Blinded by Science! (Science news update: 21 July 2022)
CDC and FDA claim that the effects of the COVID mRNA vax on reproductive health are rare. They lied.
The effect of BNT162b2 SARS-CoV-2 mRNA vaccine on menstrual cycle symptoms in healthy women
Int J Gynaecol Obstet, 2022 Jul 20. doi: 10.1002/ijgo.14356. Online ahead of print.
Abstract
Objective: To investigate the impact of the SARS-CoV-2 mRNA BNT162b2 vaccine on women's menstural cycle.
Methods: In this questionnaire-based cross-sectional study, we assessed menstrual pattern and changes of women who completed the SARS-CoV-2 mRNA BNT162b2 vaccine three months before and after receiving the vaccine. Included were women aged 18-50 without known gynecological comorbidities who regularly monitor their menstruation through electronic calendars. All participants competed a detailed questionnaire on their menstrual symptoms including information on any irregular bleeding. To minimize bias, each woman served as a self-control before and after vaccination. Primary outcome was rate of irregular bleeding following vaccination and secondary outcome was presence of any menstrual change, including irregular bleeding, mood changes or dysmenorrhea following the vaccine.
Results: A total of 219 women met the inclusion critieria. Of them, 23.3% (n=51) experienced irregular bleeding following the vaccine. Almost 40% (n=83) of study participants reported any menstrual change following vaccination. Parity was positively associated with irregular bleeding with 50% (n=26) of those suffering from irregular bleeding being multiparous as compared to only 31.5% (n=53) of women with no irregular bleeding (nulliparous 46% vs 60%, multiparous 50% vs 31%, rest 4% vs 8%, p=0.049). The presence of medical comorbidities was also significantly higher among patients who experienced irregular bleeding (20.0% vs 6.0%, p=0.003).
Conclusion: Our study shows relatively high rates of irregular bleeding and menstrual changes after receiving the SARS-CoV-2 mRNA BNT162b2 vaccine. Further research is needed to confirm our findings and to better characterize the magnitude of change and any possible long term implications.
Results from the Full Article:
All participants completed the two-dose series of the BNT161b2 mRNA vaccine with a mean interval of 22.1±5.1 days between doses. Distribution of baseline menstrual characteristics is shown in Table 2.
The average menstrual cycle length before the vaccine was 29.4±7.6 days with duration of menses of 5.0±1.3 days.
Of the 219 women who participated in the study, 23.3 % (n=51) experienced irregular bleeding following the vaccine. Of them, 39.2% (n=20) and 60.8% (n=31) reported irregular bleeding after the first and second dose of the SARS-CoV-2 BNT162b2 mRNA vaccine, respectively.
Of the 51 women who experienced irregular bleeding, 66.7% (n=34) reported irregular bleeding that preceded their estimated menstrual date (mean 9.9 ± 3.0 days) and 33.3% (n=17) reported a delay in their expected menstrual date (mean 12.3±6.3 days). Irregular bleeding was reported as light in its severity in 47% (n=24), moderate in 21.6% (n=11) and heavy in 31.4% (n=16). Among those who reported irregular bleeding, 31.4 % (n=16) reported persisted irregular bleeding duri ng the three-month period following vaccination, whereas 68.6% (n=35) reported a transient change which did not continue throughout the study period. Thirty-seven percent (n=83) of study participants reported any menstrual change (including irregular bleeding, mood changes or dysmenorrhea) following vaccination.
Nearly 68% (n=55) of study participants reported dysmenorrhea following vaccination. Amongst them, 47.3% (n=26) reported new-onset dysmenorrhea or increase in the severity of pre-existing dysmenorrhea.
Other menstrual symptoms were reported by 55.5% (n=45) of study participants after receiving the vaccine, including: abdominal pain (n=24); pelvic pain (n=11); appearance of new acne (n=11); breast tenderness (n=4); hot flushes (n=1).
Mood changes associated with menstruation after the vaccine (that were not reported before the vaccine) were reported by 9.6% (n=21) of women.
Covid-19 vaccination BNT162b2 temporarily impairs semen concentration and total motile count among semen donors
Andrology, 17 June 2022
In a retrospective, longitudinal, multicenter study published in Andrology, the effects of the COVID-19 BNT162b2 vaccine on semen parameters were assessed in 37 semen donors at different time points before and after vaccination. A selective decrease in sperm concentration and total motile count was reported 75–125 days after vaccination (P = 0.01 and P = 0.007, respectively) compared with pre-vaccination levels. Normal levels of these parameters were recovered ≥145 days post-vaccination.
(My note: This issue is critical, as sperm counts in “western “countries have decreased 52% from 1973 to 2011).
Who is Robert Malone is a reader-supported publication. To receive new posts and support my work, consider becoming a free or paid subscriber.
Menstrual cycle disturbances after COVID-19 vaccination
Women’s Health (Lond) 2022 Jan DOI: 10.1177/17455057221109375
Abstract
Introduction: After COVID-19 vaccination, women of reproductive age reported changes in their menstrual cycle.
Materials and methods: A retrospective study was carried out after a survey on social networks that included women aged 18-41 years with normal cycles according to International Federation of Gynecology and Obstetrics and who were vaccinated (complete schedule for two doses, except J&J/Janssen or incomplete with a single dose). Women with following conditions were excluded: pregnant or lactating women; history of diseases that cause menstrual irregularities or early menopause: anorexia, bulimia, polycystic ovary syndrome, hypothyroidism, obesity, or low weight; hysterectomized or oophorectomized patients; and high performance athletes.
Results: Overall, 950 women completed the survey between July and September 2021. In total, 408 women met the inclusion criteria, and 184 reported the following characteristics: frequency (normal 43.47%, infrequent 25%, and frequent 31.53%), regularity (regular 51.08%, irregular 42.93%, and absent/amenorrhea 5.97%), duration (normal 65.21%, prolonged 26.08%, absent/amenorrhea 8.69%), and volume (heavy 41.84%, light 20.65%, and absent/amenorrhea 6.52%).
Conclusions: SARS-CoV-2 infection and COVID-19 vaccination can influence the menstrual cycle and cause alterations.
Bad news for Paxlovid? Coronavirus can find multiple ways to evade COVID-19 drug
Lab studies identify resistance mutations in SARS-CoV-2’s protease, and some circulating variants have them
29 JUN 2022
A bevy of new lab studies shows the coronavirus can mutate in ways that make it less susceptible to the drug, by far the most widely used of the two oral antiviral drugs authorized to treat COVID-19 in the United States. Researchers have found some of those mutations in variants already circulating in infected people, raising fresh concerns that physicians could soon lose one of their best therapies for fighting COVID-19.
Taken together, the studies show that “when you put pressure on the virus it escapes,” says David Ho, a virologist at Columbia University who was among the first to document drug resistance mutations in HIV some 3 decades ago. Ho was not involved with the new studies but is conducting similar work on SARS-CoV-2. Although the newly identified mutations have yet to become widespread, Ho and many other scientists think it’s only a matter of time. “Given the amount of infections out there, it’s going to come,” Ho says.
The resistance studies come on the heels of other recent concerns about Paxlovid, which in the United States remains restricted to use in people with risk factors making them more likely to develop severe COVID-19. Confirming anecdotal reports widely reported by media, several studies have found a small percentage of infected people who receive the normal 5-day course initially feel better, only to have their symptoms rebound. And questions have grown about whether Paxlovid helps those who aren’t at high risk of serious disease—Pfizer earlier this month halted a large trial of the drug in standard risk COVID-19 patients because it was failing to show statistically significant protection against death or hospitalization.
Pfizer Reports Additional Data on PAXLOVID™ Supporting Upcoming New Drug Application Submission to U.S. FDA
Tuesday, June 14, 2022 - 04:30pm
In the EPIC-SR study of PAXLOVID™(nirmatrelvir [PF-07321332] tablets and ritonavir tablets), the novel primary endpoint of self-reported, sustained alleviation of all symptoms for four consecutive days was not met, as previously reported
Data from standard-risk patients, both vaccinated and unvaccinated, while not all statistically significant, are supportive of efficacy data observed in EPIC-HR study and will be included in upcoming NDA submission to U.S. FDA for high-risk patients
Pre-specified secondary endpoint resulted in a nominally significant 62% decrease in COVID-19-related medical visits per day across all patients, relative to placebo
In a sub-group analysis, non-significant 57% reduction in hospitalizations and death observed in PAXLOVID-treated vaccinated patients with at least one risk factor for severe COVID-19
Pfizer to cease enrollment into the EPIC-SR trial due to low rate of hospitalization or death in the standard-risk population; will continue to evaluate treatment in populations with high unmet need.
ERGO: Paxlovid trial stopped for lack of efficacy in standard risk patients. For the POTUS, that means the use of Paxlovid is probably unwarranted.
The data was in: Nov 2020. The Cochrane Database Syst Rev - the gold standard of meta-analysis was and is clear.
None of this mask wearing was science based. What our public health service has done to us, our children is obscene. There needs to be accountability.
Physical interventions to interrupt or reduce the spread of respiratory viruses
Meta-Analysis: Cochrane Database Syst Rev
.020 Nov 20;11(11):CD006207. doi: 10.1002/14651858.CD006207.pub5.
Authors' conclusions: The high risk of bias in the trials, variation in outcome measurement, and relatively low compliance with the interventions during the studies hamper drawing firm conclusions and generalising the findings to the current COVID-19 pandemic. There is uncertainty about the effects of face masks. The low-moderate certainty of the evidence means our confidence in the effect estimate is limited, and that the true effect may be different from the observed estimate of the effect. The pooled results of randomised trials did not show a clear reduction in respiratory viral infection with the use of medical/surgical masks during seasonal influenza. There were no clear differences between the use of medical/surgical masks compared with N95/P2 respirators in healthcare workers when used in routine care to reduce respiratory viral infection. Hand hygiene is likely to modestly reduce the burden of respiratory illness. Harms associated with physical interventions were under-investigated. There is a need for large, well-designed RCTs addressing the effectiveness of many of these interventions in multiple settings and populations, especially in those most at risk of ARIs.
Here are conclusions regarding-
Medical/surgical masks compared to no masks
We included nine trials (of which eight were cluster‐RCTs) comparing medical/surgical masks versus no masks to prevent the spread of viral respiratory illness (two trials with healthcare workers and seven in the community). There is low certainty evidence from nine trials (3507 participants) that wearing a mask may make little or no difference to the outcome of influenza‐like illness (ILI) compared to not wearing a mask (risk ratio (RR) 0.99, 95% confidence interval (CI) 0.82 to 1.18. There is moderate certainty evidence that wearing a mask probably makes little or no difference to the outcome of laboratory‐confirmed influenza compared to not wearing a mask (RR 0.91, 95% CI 0.66 to 1.26; 6 trials; 3005 participants). Harms were rarely measured and poorly reported. Two studies during COVID‐19 plan to recruit a total of 72,000 people. One evaluates medical/surgical masks (N = 6000) (published Annals of Internal Medicine, 18 Nov 2020), and one evaluates cloth masks (N = 66,000).
The data and randomized clinical trials are extensive. Please read the full conclusions in the link to the PDF below. My literature review conducted today turned up no new studies that change the Cochrane meta analysis conclusions.
One can only conclude that we have a major problem with group think at the CDC. There are no real randomized clinical trials or data to support the use of surgical or cloth masks. How come the CDC did not know this in 2020? How come they do not know this now?
What have “we” done to our children?
You think this is over?
Why is the Headstart STILL masking our toddlers and children?
Why is Los Angeles putting an indoor mask policy back in place?
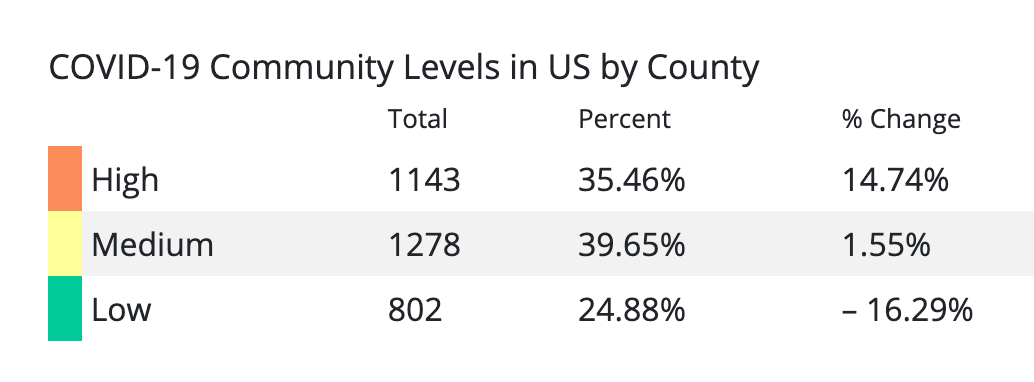
Why does the CDC still want face masks worn in areas of COVID high incidence? The CDC lists 35% of the US population in a high incidence area.
To point out the obvious, this mask wearing and other crazy policies, such as lockdowns (quarantines of our population) are not going to end with COVID-19. Soon masks will be required for the upcoming influenza season, for respiratory threats of all kinds. Soon, we will all be told to stay at home - to quarantine for a severe flu outbreak.
Folks - our immune systems need to be primed and educated. We need to be exposed to viruses, allergens, bacteria… dirt. This is what builds a strong immune system. It is why kids in the country, kids who play and work outdoors, kids who are around other kids - don’t get as sick as adults.
The CDC acts like the over-protective parent of a sickly child. A parent who is doing more harm than good.
Time for this crazy mass-psychotic event, which has been orchestrated by public health and the CDC to end. Human life on this planet needs to return to normal.
Is there a COVID medical emergency? No. So let’s just stop pretending that the declaration of medical emergency that was just renewed has anything to do with public health.
Important to remember that the HHS considers anyone in the hospital who has tested positive for SARS-CoV-2 antigen or genetic information (eg. PCR) to be a COVID patient. Which is highly misleading at best, and disinformation at worst.




Just more proof our government along with other governments are knowingly, willingly and with intent committing crimes against humanity for the power of Gov over the populace 🤬
Remember all the way back to last year when talking about such things was dangerous misinformation?
https://simulationcommander.substack.com/p/government-is-a-relentless-freedom
"The COVID-19 vaccines have been shown to cause infertility,”
COVID VACCINE TEMPORARILY IMPAIRS ABILITY OF SPERM TO SWIM, ISRAELI STUDY REVEALS