Myopericarditis and mRNA SARS-CoV-2 vaccines - FDA and CDC administrators lied, and people (particularly children) died.
US Government political appointees and career employees knew very early, withheld the information, and blocked the minimal informed consent required by EUA
Some assert that the Cures Act is what enabled the US Federal Government to bypass informed consent regarding the gene therapy-based COVID vaccines.
Read that again.
“Except where it is not feasible, it is contrary to the best interests of such human beings, or the proposed clinical testing poses no more than minimal risk to such human beings and includes appropriate safeguards as prescribed to protect the rights, safety, and welfare of such human beings”
How does the FDA define minimal risk?
Minimal risk means that the probability and magnitude of harm or discomfort anticipated in the research are not greater in and of themselves than those ordinarily encountered in daily life or during the performance of routine physical or psychological examinations or tests.
US Government officials have repeatedly refused to provide required full informed consent for either the experimental use authorized “vaccine” products or for those participating in the clinical research and development phase of these products. One hypothesis for why this has been the case relates to the above absolution clause buried in the “21st Century “Cures” act”. If this is indeed the case, then it would seem that the FDA, CDC, DoD etc. would have a strong interest in defending the position that the "genetic vaccines” pose minimal risk to human beings. Perhaps that is why there is a pattern of chronic denialism of what are clearly significant adverse events such as myopericarditis?
Various internet researchers including Dr. Sasha Latypova and “Chief Nerd” have asserted or implied that this Cures act language is the clause which has empowered the US Government to bypass the international consensus on requirement for informed consent for medical experimentation on human subjects, all medical procedures, and administration of unlicensed medical products. But this does not appear to be the case, even though this assertion has been widely circulated on social media.
The US Government has functionally acted as the product development “sponsor” during the development and testing of the SARS-CoV-2 genetic “vaccines” under “Operation Warp Speed”. Therefore it is the responsibility of the relevant US Government employees/administrators to ensure compliance with all applicable laws and regulations involving these products during the experimental clinical research development and testing phase. Furthermore, under Emergency Use Authorization (EUA), these products are not authorized for marketing by the manufacturers, which have been acting as agents of the US Government under contract. All responsibility for the products during while under EUA vests with the relevant US Government agencies (primarily FDA, also CDC) and their administrative/Senior Executive Service and politically appointed leadership.
The FDA appears to assert, in part, that their authorization to bypass informed consent flows from a separate law, specifically Public Law 115-92 which was enacted by the115th Congress on Dec. 12, 2017, although for the life of me I cannot tell which aspects of this byzantine act confer the right to bypass true informed consent. It may be that this is another example of liberal application of administrative interpretation rather than clear congressional intent.
Here is the text relating to informed consent from the FDA guidance on Emergency Use Authorization:
b. Information for Recipients
Although informed consent as generally required under FDA regulations45 is not required for administration or use of an EUA product, section 564 does provide EUA conditions to ensure that recipients are informed about the MCM they receive under an EUA. For an unapproved product (section 564(e)(1)(A)(ii)) and for an unapproved use of an approved product (section 564(e)(2)(A)), the statute requires that FDA ensure that recipients are informed to the extent practicable given the applicable circumstances:
That FDA has authorized emergency use of the product;
Of the significant known and potential benefits and risks associated with the emergency use of the product, and of the extent to which such benefits and risks are unknown;
That they have the option to accept or refuse the EUA product and of any consequences of refusing administration of the product;46 and
Of any available alternatives to the product and of the risks and benefits of available alternatives.
Therefore, FDA recommends that a request for an EUA include a “Fact Sheet" for recipients that includes essential information about the product. In addition to the required information, the agency recommends that the content of the Fact Sheets for recipients include the following information:
Product name and explanation of the intended use of the product
A description of the disease/condition;
A description of items to discuss with a health care provider and adverse event information, including contact information for how to get more information and for reporting adverse reactions; and
Dosing information (if applicable), including specific instructions for home use or preparation (if applicable).
FDA recommends that recipients be given as much appropriate information as possible given the nature of the emergency and the conditions of the authorization.47
<Note: For the EUA recently granted for the “Booster” vaccines, there was no attempt to justify the “nature of the emergency”. Probably because there is no public health emergency, either via declaration from the Secretary of HHS or DoD (or POTUS), nor are there data indicating the current presence of a public health emergency.>
Ordinarily, FDA expects that some written form of information will be given to recipients with the MCM, similar to the Fact Sheet for health care professionals or authorized dispensers. FDA recognizes that these Fact Sheets, like those for health care professionals or authorized dispensers, will generally be brief. To ensure that individuals of varying educational levels comprehend the information provided, FDA recommends that all written information be stated in the simplest language possible using techniques to improve health literacy.48 In addition, translations to other languages may be appropriate if practicable.49 FDA recognizes that some flexibility may be needed for health care providers or authorized dispensers to make minor, nonsubstantive changes to the fact sheets for recipients such as adding local contact information, using specific letterhead or minor format changes.
Did the FDA comply with the “informed consent waiver” clauses of its own guidance on EUA? Let’s review the language:
That FDA has authorized emergency use of the product;
<Yes, this was certainly disclosed to the public>
Of the significant known and potential benefits and risks associated with the emergency use of the product, and of the extent to which such benefits and risks are unknown;
<No, actually there was not a clear and accurate statement and summary of known, potential, and unknown risks provided to citizens>
That they have the option to accept or refuse the EUA product and of any consequences of refusing administration of the product;
<No, this was not stated and in fact, the government clearly acted to directly or indirectly mandate receipt of the EUA products>
Of any available alternatives to the product and of the risks and benefits of available alternatives.
<No, the FDA actively blocked and disparaged available alternatives to the products in the form of various early drug treatment agents and products>
Based on the above analysis alone, I assert that prior and current EUA for the genetic SARS-CoV-2 vaccine products are illegal and represent arbitrary and capricious regulatory overreach.
Does the FDA have authority to issue a new EUA, or to maintain the existing EUA?
Here is the relevant guidance language:
A. EUA DECLARATION JUSTIFYING EMERGENCY USE
1. Determinations to Support an EUA Declaration
Before FDA may issue an EUA, the HHS Secretary must declare that circumstances exist justifying the authorization (section 564(b)(1)). This declaration (referred to in this guidance as an “EUA declaration”),12 must be based on one of the following actions:
A determination by the Secretary of Homeland Security that there is a domestic emergency, or a significant potential for a domestic emergency, involving a heightened risk of attack with a CBRN agent(s);13
A determination by the Secretary of Defense that there is a military emergency, or a significant potential for a military emergency, involving a heightened risk to United States military forces of attack with a CBRN agent(s);14
A determination by the Secretary of HHS that there is a public health emergency, or a significant potential for a public health emergency, that affects, or has a significant potential to affect, national security or the health and security of United States citizens living abroad, and that involves a CBRN agent or agents, or a disease or condition that may be attributable to such agent(s);15 or
The identification of a material threat, by the Secretary of Homeland Security pursuant to section 319F-2 of the Public Health Service (PHS) Act, that is sufficient to affect national security or the health and security of United States citizens living abroad.16
After the Secretary of HHS issues an EUA declaration based on one of these four determinations, and after consulting (to the extent feasible and appropriate given the applicable circumstances) with the Assistant Secretary for Preparedness and Response (ASPR), the Director of the National Institutes of Health (NIH), and the Director of CDC,17 the Commissioner may authorize the emergency use of an unapproved product or an unapproved use of an approved product, provided that other statutory criteria are met.
In appropriate circumstances, an HHS EUA declaration may support issuance of more than one EUA. For example, based on an HHS EUA declaration that circumstances exist to justify the authorization of emergency use of diagnostics for a specified biological agent, FDA may authorize emergency use for multiple diagnostic tests to meet the need, provided that each EUA meets the statutory criteria for issuance.
2. Termination of an EUA Declaration
When an EUA declaration is terminated, then any EUA(s) issued based on that declaration will no longer remain in effect.18 The HHS Secretary’s EUA declaration will terminate on the earlier of: (1) a determination by the HHS Secretary that the circumstances that precipitated the declaration have ceased (after consultation as appropriate with the Secretary of Homeland Security or the Secretary of Defense), or (2) a change in the approval status of the product such that the authorized use(s) of the product are no longer unapproved (section 564(b)(2)). For example, an EUA issued to allow an unapproved use of an approved product may no longer be needed if that product is later approved by FDA for the use permitted by the EUA.
<BOTH of these termination criteria have now been met for the mRNA-based “vaccines” from Moderna and Pfizer/BioNTech. 1) The declaration of Public Health Emergency was terminated by the POTUS (and Sec HHS) on May 11, 2023. 2) The approval status of the mRNA-based “vaccines” has changed- they are now approved. Just because the manufacturers refuse to market the approved versions in the USA (apparently due to liability issues) the FDA has in fact approved them for marketing. Therefore, based on the FDA’s own guidance, the EUA for these products is to be terminated. Issuance of a NEW EUA for the “booster” products is illegal, as there is no Public Health Emergency at this time.>
Before an EUA declaration terminates, the Secretary of HHS must provide advance notice that is sufficient to allow for the disposition of an unapproved product, and of any labeling or other information provided related to an unapproved use of an approved product (section 564(b)(3)).19
<That notice was provided, 180 days post May 11, 2023 was administratively granted to the FDA unilaterally by the FDA. Therefore, based on the sum of the above, I assert that (at a minimum) all existing EUA granted in the context of the expired COVID declaration of public health emergency shall be terminated consistent with federal law and the FDA’s own guidance when those 180 days expire- Tuesday, November 7, 2023>
What did the FDA and HHS know about the risk of Myopericarditis associated with the gene therapy-based mRNA COVID “vaccines”, and when did it know it?
In a journalistic tour de force, Zachary Stieber, Lia Onely and their colleagues at the Epoch Times have recently provided extensive documentation of who in the USG knew what and when regarding the association of myopericarditis with the mRNA-based COVID “vaccines”.
By Zachary Stieber, Lia Onely Sep 17, 2023, Updated: Sep 19, 2023
COVID-19 vaccines cause heart inflammation, U.S. authorities now acknowledge. But after being warned in early 2021 about a "large number" of cases among healthy, young people in Israel after COVID-19 vaccination, authorities did not immediately alert the public while also failing to detect a safety signal that was present in the United States, an Epoch Times investigation has found.
Even after deaths from myocarditis—inflammation of the heart—were reported and myocarditis was designated as a likely side effect of the shots, U.S. officials kept recommending vaccination for virtually the entire populace.
That led to millions of young people receiving a vaccine.
Many of those people suffered.
The CDC, America's public health agency, was warned by Israel on Feb. 28, 2021, about a "large number" of myocarditis cases after Pfizer COVID-19 vaccination, documents obtained by The Epoch Times show.
Internally, the warning was designated as "high" importance and set off a review of U.S. data. The review found 27 reported cases in the United States, according to a U.S. government memorandum dated March 9, 2021. The incidence rate was low, but "missing and incomplete data make it challenging to assess causation," the memo stated. The U.S. Food and Drug Administration (FDA), it said, "has not made a final determination regarding the causality."
Weeks later, neither the CDC nor the FDA had alerted the public to the issue, even after the death of a previously healthy 22-year-old Israeli woman and briefings from Israeli officials and U.S. Department of Defense (DOD) researchers.
Like Israel, the DOD was recording a higher-than-expected number of myocarditis cases. Patients were mostly young, healthy males.
The CDC met with military officials twice behind closed doors in April 2021. Military officials presented data during at least one of the meetings to the CDC. That presentation, which has never been released to the public, "included our preliminary patient data and analysis that suggested to us that myocarditis was indeed a possible side effect to the messenger RNA COVID-19 vaccines (within the US military)," Dr. Jay Montgomery, one of the presenters, told The Epoch Times via email.
On April 27, 2021, after the meetings, then-CDC Director Dr. Rochelle Walensky finally spoke about the matter in public, during a White House briefing.
Dr. Walensky said "we have not seen any reports" of myocarditis after vaccination. That's false, according to CDC data—the agency received 141 reports of myocarditis in the Vaccine Adverse Event Reporting System (VAERS) by the end of March 2021. Another 24 cases were recorded in the Vaccine Safety Datalink, a second system run by the CDC.
Additionally, before the briefing, Dr. Walensky was copied on multiple threads discussing myocarditis and a related condition, pericarditis, including a thread about doctors in California seeing the cases, internal emails obtained by The Epoch Times show. She responded to one of the threads, saying the information was "super helpful."
"We have not seen a [safety] signal," Dr. Walensky also told reporters during the briefing, "and we’ve actually looked intentionally for the signal in the over 200 million doses we’ve given."
Mr. Stieber has kindly also provided a detailed timeline of these and the following events which the Epoch Times’ team extensively documented and analyzed in the parent article.
Timeline: COVID-19 Vaccines and Myocarditis: A timeline of COVID-19 vaccines and myocarditis.
The results of their work are damning. The FDA and CDC were clearly aware of the scope and nature of this adverse event, and also clearly avoided disclosing what they knew to the citizens of the US and world for as long as possible. Their actions in this are the direct opposite of the informed consent information which they were required to provide by Federal law and the FDA’s own EUA guidance.
FDA and CDC administrators lied, and people (particularly children) died.
These individuals must be held accountable for their fraudulent actions. This is actual disinformation, false information intentionally distributed for political purposes. These actions clearly meet the criteria which DHS secretary Mayorkas and his colleagues have defined as domestic terrorism.
And still the FDA and CDC assert that these products are safe and effective. And still schools, athletic programs, and Universities insist that young people receive these products or they will not be allowed to participate in educational, professional certification or sports activities.
What are the practical, clinical impact of these policies? For the most recent update, I bring your attention to the comprehensive work of Mr. Ed Dowd and his colleagues and their most recent report on Death & Disability Trends, Ages 15-44: Cardiovascular Diseases, which relies on data from the UK Office of National Statistics (ONS).
In this study, we investigate the UK trends in death rates and disabilities for diseases of the cardiovascular system for individuals aged 15 to 44. We compute excess death rates and excess disability claims, which are the difference between observed deaths/disability rates and a given baseline for expected death/disability rates. We measure changes in the behaviour of morbidity and mortality before the Covid-19 pandemic with the postpandemic period for diseases of the cardiovascular system. We show a large increase in morbidity (disabilities) and mortality due to diseases of the cardiovascular from 2021. The increase in disability claims is consistent with the increase in excess deaths, and both are highly statistically significant (black swan events). The results indicate that from 2021 a novel phenomenon leading to increased cardiovascular deaths and disabilities appears to be present in individuals aged 15 to 44 in the UK.
We can observe that deaths per year from cardiovascular diseases have been trending lower from 2010 to 2019, with a significant downward slope. In 2010 the deaths rate was 10 per 100,000, in 2019 it was around 8 per 100,000, a 20% drop. The death rate increased in 2020 to about 9 per 100,000 and then again in 2021 to 10 per 100,000. In 2022 the death rate increased again to about 11 per 100,000, a level that is 10% higher than observed in 2010. The death rate in 2022 was about 3 deaths per 100,000, higher than the 2015-2019 average. When translating these numbers into the absolute number of deaths for diseases of the circulatory system, shown in Figure (right), we can observe that the 5-year average deaths from 2015 to 2019 was about 1800 deaths. In 2000, cardiovascular deaths were about 2,000, 200 more than the prior 5-year average. In 2021 there were about 2300 deaths (500 more than the 2015-2019 average) and in 2022, 2500 (700 more than the 2015-2019 average).
Disability PIP Clearances for new Claims from Cardiovascular Diseases
The analysis we present here refers to clearances from new claims to the system. It should be noted that clearances refer to decisions made, which can be positive or negative. The fraction of positive clearances (that lead to a grant allowance) is shown to be stable over time at a rate of about 40%. On our website, we present the analysis of trends in PIP clearances for the different body systems which include interactive charts where the user/researcher can change body system, age of the individuals and trend metric. The figure shows yearly clearances for new claims to the Personal Independence Payment (PIP) system in the UK for the cardiovascular system for ages 16 to 44. The dotted line refers to the 2016 to 2019 average yearly number of new claims.
Analysis of Excess Death & Disability Rates for Cardiovascular Diseases, Ages 15-44
Analysis of Excess Adjusted Death Rates from Cardiovascular Diseases
In this section we investigate the trends in excess deaths from diseases of the circulatory system (cardiovascular diseases), with ICD10 codes ranging from I00 to I99, in England and Wales, for the 15-44 age group. We also compare excess deaths for males and females and compute excess disability claims. In Figure below (left) we can observe that the excess deaths rates from cardiovascular diseases rose by about 13% in 2020, 30% in 2021 and about 44% in 2022. On the other hand, the excess mortality for all registered deaths UK was about 5% in 2020, 15% in 2021 and 10% in 2022. The drop in excess mortality for all registered deaths from 2021 to 2022 was not mirrored in a drop in cardiovascular deaths. The opposite occurred, with a sharp acceleration in excess deaths due to cardiovascular diseases. When looking at excess deaths for cardiovascular diseases, the Z-score in 2020 was around 3, indicating that prior to the start of the vaccinations there was already a signal pointing to an increase in cardiovascular deaths. That trend however accelerated substantially in 2021 and 2022 where we observe Z-scores of around 7.5 and 10.5, respectively. These are extreme events that we believe need a thorough investigation.
Our analysis shows that the excess death rates from cardiovascular diseases rose by about 13% in 2020, 30% in 2021, and about 44% in 2022. The excess mortality from cardiovascular deaths in 2021 and 2022 are highly statistically significant with Z-scores of 7.5 and 10.5, respectively. These are very strong signals. These signals are corroborated by similar findings when measuring rises in the fraction of deaths from cardiovascular diseases relative to all other deaths with classified causes (see full report above).
Analysis of Excess Adjusted Death Rates from Cardiovascular Diseases (Males & Females)
We now compare excess deaths rates from cardiovascular diseases for males and females aged 15-44, as shown in the Figure below. When looking at deaths attributed to the circulatory system for males and females, shown in the figure below, we observe that in 2020 and 2021 both had similar outcomes in excess mortality (deviation from trend) as well as the respective Z-scores (statistical significance). However, we also observe that in 2022 men suffered much worse outcomes than women, with men experiencing a 56% deviation from trend, compared to about 28% for women.
When comparing outcomes for men and women, we observe that they had similar rises in deaths from cardiovascular diseases in 2020 and 2021. However, in 2022, men suffered much worse outcomes than women, with men experiencing a 56% deviation from trend, compared to about 28% for women. Further investigation is needed. We speculate that one of the factors contributing to this difference may be men doing more physical exercise than women, which increases the probability of fatal outcomes, once the heart muscle has suffered prior (mild) injury.
Conclusions
The results shown in table above indicate that there was a significant rise in both disability claims and deaths in the 15-44 age group in the UK. We observe that there was a 6.6 per 100,000 excess rate of disability claims, and only a rate of 2.2 per 100,000 excess deaths in 2021. These rates rose in 2022 with 13.2 per 100,000 disability claims and 3.08 per 100,000 excess deaths. This compares with a baseline pool of possible injured individuals of 2,800 per 100,000 reported on the paper by Buergin et al. We also observe that the relative changes in disabilities were more than double the equivalent rises in deaths, which points towards the risk of higher cardiovascular deaths in the coming years as these conditions remain unresolved. The observations above point to a worrying picture that we might see an even greater acceleration of cardiovascular deaths and disabilities in the coming years, which makes the investigation of the underlying causes of upmost importance. We are currently in the process of pursuing further investigations into this issue in more detail. In particular, we aim to analyse the trends in deaths and disabilities for the most common individual ICD10 causes within the circulatory system, in order to have insights into the underlying phenomenon of action.
And still the FDA and CDC insist that these products are “safe and effective”, and recommend them for all aged five years and over.
Who will answer for this? When will there be changes in policy, or even a modicum of common sense? By what mechanism will there be any accountability?
“Who is Robert Malone” is a reader supported publication. Please consider a paid subscription if you can afford it:
Timeline: COVID-19 Vaccines and Myocarditis
(From the Epoch Times Timeline)
2020
Sept. 22, 2020: U.S. Centers for Disease Control and Prevention (CDC) identifies myocarditis as an adverse event of special interest, or a potential side effect.
Oct. 30, 2020: U.S. Food and Drug Administration (FDA) identifies myocarditis as an adverse event of special interest.
December 2020: One case of pericarditis reported to the U.S. Vaccine Adverse Event Reporting System (VAERS), which is co-managed by the CDC and FDA.
Dec. 18, 2020: FDA authorizes the U.S. government-backed Moderna vaccine for Americans 18 and older.
2021
2021: Myocarditis cases spike in the U.S. military.
January 2021: 28 cases of myocarditis, pericarditis, or myopericarditis reported to VAERS.
January 2021: First U.S. military member experiences postvaccination myocarditis, according to a study published months later.
January 2021: First cases of postvaccination myocarditis recorded in Israel.
January 2021: VAERS report processing is delayed due to unexpected spike in reports.
February 2021: 64 cases of myocarditis, pericarditis, or myopericarditis reported to VAERS, including two deaths.
Feb. 1, 2021: Israeli teenager is hospitalized with myocarditis after Pfizer vaccination, doctors say.
Feb. 18, 2021: Safety signal for myocarditis triggered in VAERS using CDC-endorsed method called Proportional Reporting Ratio.
Feb. 19, 2021: Safety signal for myocarditis triggered in VAERS using another method called Fisher’s Exact Test.
Feb. 24–25, 2021: CDC meets with its advisers but does not discuss COVID-19 vaccines.
Feb. 27, 2021: FDA authorizes Johnson & Johnson's COVID-19 vaccine.
Feb. 28, 2021: Israeli officials privately alert CDC to "a large number of reports of myocarditis, particularly in young people, following the administration of the Pfizer vaccine."
Feb. 28, 2021: 57 cases of myocarditis or pericarditis within seven days of vaccination in Pfizer's database in document given to the FDA in April 2021 and not revealed to the public until November 2021.
March 2021: 54 cases of myocarditis, pericarditis, or myopericarditis reported to VAERS.
March 1, 2021: CDC officials disclose (pdf) that two postvaccination cases were identified in the Vaccine Safety Datalink (VSD), another CDC-run system.
March 2, 2021: CDC recommends Johnson & Johnson's COVID-19 vaccine for adults.
March 3, 2021: Israeli authorities meet with hospital officials to discuss postvaccination heart problems.
March 4, 2021: Israeli officials confirm they're investigating postvaccination pericarditis.
March 5, 2021: FDA holds meeting with its advisers. Myocarditis is not discussed.
March 6, 2021: Rutgers University becomes first major US school to announce COVID-19 vaccine mandate.
March 8, 2021: Australian health officials contact CDC about U.S. myocarditis cases.
March 9, 2021: U.S. internal memorandum says Israel received around 40 reports of postvaccination myocarditis. U.S. officials say some postvaccination cases were reported in the United States and acknowledge issues with passive surveillance such as underreporting. "Thus, FDA has not made a final determination regarding the causality between myopericarditis and the mRNA COVID-19 vaccines," the memo stated.
March 20, 2021: First postvaccination myocarditis case report is published in the literature.
March 20, 2021: Pfizer contract with South Africa (pdf) says that "there may be adverse effects of the vaccine that are not currently known."
March 31, 2021: Second postvaccination myocarditis case report published.
March 31, 2021: First death from postvaccination myocarditis reported in Israel. The deceased was a 22-year-old previously healthy woman.
April 2021: 158 cases of myocarditis, pericarditis, or myopericarditis reported to VAERS.
April 2021: CDC holds multiple public meetings with no discussion of myocarditis.
April 1, 2021: Israel has received 84 reports of postvaccination myocarditis or pericarditis, news outlet reports.
April 2, 2021: U.S. military officials, CDC meet on postvaccination myocarditis cases.
April 5, 2021: Israeli officials brief the CDC on postvaccination myocarditis cases.
April 5, 2021: Canada reports first case of postvaccination pericarditis.
April 7, 2021: U.S. government call covers myocarditis cases recorded in military members after vaccination.
April 10, 2021: CDC-funded Clinical Immunization Safety Assessment program reviewed postvaccination myocarditis cases, email shows.
April 12, 2021: U.S. military officials brief CDC on postvaccination myocarditis cases.
April 12, 2021: Canada reports first case of postvaccination myocarditis.
April 13, 2021: U.S. military has submitted manuscripts on myocarditis cases to two journals, CDC official says.
April 13, 2021: CDC, FDA advise pause in administration of Johnson & Johnson's vaccine due to six cases of blood clotting.
April 15, 2021: Two otherwise healthy adults hospitalized with chest pain and diagnosed with myocarditis after Moderna vaccination, CDC adviser tells agency.
April 17, 2021: CDC official tells adviser there have been reports of myocarditis after COVID-19 vaccination "but we aren't observing any clear indication of a safety signal."
April 20, 2021: Three cases of myocarditis after second Pfizer dose in Idaho, official tells CDC.
April 23, 2021: United States lifts recommended pause on Johnson & Johnson's vaccine.
April 23, 2021: Israeli Ministry of Health report on postvaccination myocarditis identifies two deaths, Israeli media report. The second deceased was a previously healthy 35-year-old man. Among men aged 18 to 30, there is a one in 20,000 probability of developing myocarditis, the report found. "It seems that these events can be a signal of a possible connection to the vaccine," the committee said. The findings were sent to the FDA.
April 26, 2021: U.S. military officials are tracking 14 cases of myocarditis following messenger RNA vaccination, Military.com reports.
April 26, 2021: CDC Director Dr. Rochelle Walensky says CDC "aware of" cases in the military. She says "we have not seen any reports of those" and that "we have not seen a signal" in CDC databases.
April 27, 2021: CDC officials privately acknowledge that processing of VAERS reports is "taking longer than usual."
April 27, 2021: CDC officials say 24 cases of myocarditis were identified in VSD.
April 27, 2021: U.S. military official warns that pausing administration of the Pfizer and Moderna vaccines "will have an adverse impact on US/CA vaccination rates."
April 28, 2021: France detects safety signal for postvaccination myocarditis.
April 28, 2021: CDC director receives notes from discussion with military on myocarditis cases.
April 29, 2021: First cases of pericarditis after COVID-19 vaccination reported in the literature.
April 30, 2021: FDA receives Pfizer report noting myocarditis cases in Pfizer's database.
May 2021: 487 cases of myocarditis, pericarditis, or myopericarditis reported to VAERS, including four deaths.
May 2021: CDC forms a team to review medical records for reported cases of postvaccination myocarditis.
May 5, 2021: CDC meets with advisers but doesn't discuss COVID-19 vaccines.
May 7, 2021: European Medicines Agency announces it has asked Pfizer for information on myocarditis after COVID-19 vaccination.
May 10, 2021: FDA authorizes Pfizer's COVID-19 vaccine for children aged 12 to 15. It does not mention myocarditis.
May 12, 2021: Myocarditis is not discussed during meeting on Pfizer's COVID-19 vaccine. Dr. Tom Shimabukuro, a CDC official, gives a presentation on blood clotting after Johnson & Johnson vaccination.
May 12, 2021: Dr. Walensky recommends Pfizer's vaccine for virtually all children aged 12 to 15, based on advice from advisers.
May 13, 2021: CDC officials told to direct questions on potential myocarditis cases to two top vaccine safety officials, Drs. John Su and Shimabukuro.
May 13, 2021: Same officials discuss analyzing VAERS data for myocarditis using the Proportional Reporting Ratio in heavily redacted emails. The agency says the analysis didn't actually start until 2022.
May 13, 2021: CDC adviser says "multiple people texting and email[ing] me with concerns" about myocarditis.
May 13, 2021: Children's National in Washington registers two suspected cases.
May 14, 2021: Dr. Shimabukuro seeks "experts in myocarditis."
May 16, 2021: CDC official says in email, "we are hearing quite a lot about this now, and I don't have a clear understanding of what is and has been being done."
May 17, 2021: CDC workgroup says there are "relatively few" reports of myocarditis after vaccination and that rates "have not differed from expected baseline rates."
May 17, 2021: Dr. Shimabukuro speaks with American Academy of Pediatrics officials to "centralize our coordination" with outside groups.
May 17, 2021: Dr. Su says health care providers "aren't reporting these cases to VAERS."
May 17, 2021: Dr. Su says the "myocarditis thing" is "exploding."
May 18, 2021: States across the U.S. publicly report cases of myocarditis following COVID-19 vaccination.
May 18, 2021: First case report of an American person with postvaccination myocarditis published.
May 19, 2021: CDC tells state officials it has been "closely monitoring" myocarditis and pericarditis after COVID-19 vaccination and that cases "can be serious."
May 20, 2021: Three more cases of postvaccination myocarditis at Connecticut Children's Medical Center, official tells CDC.
May 20, 2021: CDC officials hold meeting with doctors from pediatric hospitals on myocarditis cases. Slides from the meeting were provided to The Epoch Times fully redacted.
May 23, 2021: The American Heart Association says the benefits of COVID-19 vaccination for everyone eligible "enormously outweigh the rare, possible risk of heart-related complications, including inflammation of the heart muscle."
May 24, 2021: CDC workgroup acknowledges for the first time that the number of reports of postvaccination myocarditis to VAERS was higher than expected in those 16 to 24.
May 24, 2021: French Society of Cardiology calls for vaccinating heart failure patients without acknowledging possible vaccine-myocarditis connection.
May 24, 2021: Massachusetts official asks CDC for "messaging" on postvaccination myocarditis.
May 25, 2021: Dr. Paul Offit, an FDA adviser, says about myocarditis and COVID-19 vaccines, "there’s every reason to think this isn’t a problem."
May 26, 2021: The New Zealand Medicines and Medical Devices Safety Authority says there's a safety signal for myocarditis and COVID-19 vaccines.
May 28, 2021: CDC says to keep vaccinating everyone eligible, or virtually all Americans 12 and older.
May 28, 2021: Case series of young, previously healthy males hospitalized with postvaccination myocarditis discloses hospitalizations happened as early as Jan. 30, 2021.
May 28, 2021: CDC says it is focusing on reported cases among those 30 and under.
May 28, 2021: Nine postvaccination myocarditis cases from one state not reported to VAERS, according to CDC.
May 30, 2021: Brighton Collaboration issues case definition for myocarditis.
June 2021: 752 cases of myocarditis, pericarditis, or myopericarditis reported to VAERS, including five deaths.
June 2021: Some experts start calling on U.S. authorities to pause COVID-19 vaccination of young, healthy people.
June 2, 2021: Israel says "there is some probability for a possible link between the second vaccine dose and the onset of myocarditis among young men aged 16 to 30" after researchers find incidence of one in 3,000 to one in 6,000 men aged 16 to 24.
June 4, 2021: U.S. researchers report seven cases in healthy young males following Pfizer vaccination.
June 10, 2021: 99 cases of myocarditis/pericarditis detected in FDA's Biologics Effectiveness and Safety Initiative database, and 1,260 cases reported in Medicare claims data, FDA official says (pdf).
June 10, 2021: CDC working to rapidly follow up on reports of myocarditis following vaccination among people aged 30 and under, CDC official says (pdf). Most cases are in young adults after a second dose.
June 10, 2021: "There’s a lack of alternative explanations" apart from vaccination given the consistency across postvaccination cases, Dr. Cody Meissner, an FDA adviser, says.
June 10, 2021: "I think the myocarditis is something that needs to be looked at closely because we’re likely seeing the tip of the iceberg," says Dr. Michael Kurilla of the U.S. National Institutes of Health, another FDA adviser. June 10, 2021: Pfizer spokesperson tells news outlets that "the benefit-risk profile of our vaccine remains positive."
June 11, 2021: European Medicines Agency announces investigation into reports of myocarditis after COVID-19 vaccination.
June 23, 2021: CDC’s safety committee says evidence now suggests a "likely association" of mRNA vaccination and myocarditis.
June 23, 2021: Number of myocarditis cases recorded in VSD rise to 75. Based on VAERS reports, CDC says number of events higher than expected after dose two in males aged 12 to 49 and females aged 12 to 29. Among children 12 to 17, 188 cases were reported within 21 days of vaccination through June 11. CDC advisers say data suggest vaccines cause myocarditis.
June 23, 2021: CDC estimates (pdf) Pfizer's vaccine will cause up to 69 myocarditis cases per million second doses but will prevent 215 hospitalizations and two deaths in males aged 12 to 17.
June 25, 2021: FDA adds warnings about "the suggested increased risks" of myocarditis and pericarditis to labels for the Moderna and Pfizer vaccines.
June 28, 2021: The U.S. Department of Veteran Affairs says privately it detected more myocarditis cases than expected.
June 29, 2021: Military researchers report 22 previously healthy members suffered myocarditis after receiving a messenger RNA vaccine. "The presentation pattern and clinical course suggest an association with an inflammatory response to vaccination," they say. The cases are among hundreds reported in the literature this month.
June 29, 2021: CDC officials say of reported cases that "the striking clinical similarities in the presentations of these patients, their recent vaccination with an mRNA-based COVID-19 vaccine, and the lack of any alternative etiologies for acute myocarditis suggest an association with immunization."
June 30, 2021: Canada adds myocarditis and pericarditis risk information to labels of Moderna and Pfizer vaccines.
July 2021: 364 cases of myocarditis, pericarditis, or myopericarditis reported to VAERS, including seven deaths.
July 3, 2021: 13 young males with postvaccination myocarditis treated at a single hospital in Washington state between April 1, 2021, and June 21, 2021, researchers report.
July 6, 2021: CDC estimates for every million doses of vaccination, dozens of cases of myocarditis can be expected, including 56 to 69 cases among 12- to 17-year-olds. The expected benefits outweigh the risks, though, according to the agency.
July 9, 2021: European Medicines Agency recommends listing myocarditis and pericarditis as side effects for the Pfizer and Moderna vaccines after identifying 221 cases after mRNA vaccination. Five of the patients died.
July 10, 2021: South Korean researchers report a 22-year-old man was killed by vaccine-induced myocarditis, the first such death reported in the literature.
July 22, 2021: CDC says it confirmed 282 postvaccination myocarditis cases in people aged 18 to 29.
July 28, 2021: Pfizer tells FDA of "important identified risk" of myocarditis and pericarditis after vaccination and discloses 17 deaths among the cases reported.
July 30, 2021: Data mining does not show a signal for myocarditis among adolescents 12 to 17, CDC and FDA say.
July 31, 2021: Australian experts recommend people be informed about possibility of myocarditis and pericarditis following vaccination.
August 2021: 311 cases of myocarditis, pericarditis, or myopericarditis reported to VAERS, including seven deaths.
Aug. 4, 2021: UK delays recommending second dose for 16- and 17-year-olds.
Aug. 10, 2021: 15 children hospitalized at Boston Children's Hospital with postvaccination myocarditis between May 1 and July 15, 2021, researchers say.
Aug. 13, 2021: Canada identifies a safety signal for postvaccination myocarditis.
Aug. 17, 2021: Death of 27-year-old man following myocarditis after COVID-19 vaccination reported by U.S. researchers. Family declined an autopsy. No non-vaccination causes identified.
Aug. 18, 2021: U.S. researchers report death of 42-year-old man with myocarditis following Moderna vaccination. No other causes identified.
Aug. 18, 2021: Dr. Walensky and other U.S. government officials call for COVID-19 vaccine boosters to be cleared due to waning effectiveness.
Aug. 19, 2021: Two deaths from myocarditis following Pfizer vaccination, Pfizer reports to the EMA.
Aug. 19, 2021: Dr. Walensky notified of UK preference for Pfizer over Moderna for adolescents.
Aug. 23, 2021: FDA approves Pfizer's vaccine for people 16 and older. Approval theoretically has a higher bar than authorization. The agency says that analyses of reported events "will not be sufficient to assess known serious risks of myocarditis and pericarditis."
Aug. 24, 2021: South Korean authorities determine a young man died from myocarditis after Pfizer vaccination.
Aug. 30, 2021: One myocarditis case and one pericarditis case occurred among vaccinated trial participants, Pfizer reveals.
Aug. 30, 2021: Of 742 reports in VAERS that met the CDC case definition of myocarditis or myopericarditis after vaccination, 701 patients required hospitalization and 18 are still hospitalized, CDC official reports (pdf). Twenty-three percent of patients whose cases were detailed in VAERS reports were not known to have recovered at the time of the report.
Aug. 30, 2021: Highest rate of reported myopericarditis cases within seven days of a shot was 71.5 cases per million second Pfizer doses among boys aged 16 or 17, according to the CDC.
Aug. 30, 2021: CDC announces (pdf) plans to follow myocarditis patients to try to assess long-term outcomes.
Aug. 30, 2021: Myocarditis and pericarditis cases in VSD rise to 115, with some patients still experiencing symptoms, CDC official says (pdf).
Aug. 30, 2021: Benefits of Pfizer's vaccine continue to outweigh the risks, CDC officials say. They estimate every million Pfizer shots among 16- to 17-year-olds will cause 73 cases of myocarditis but prevent more hospitalizations.
Aug. 30, 2021: Woman who died in New Zealand after Pfizer vaccination perished from myocarditis, an independent safety panel said.
September 2021: 377 cases of myocarditis, pericarditis, or myopericarditis reported to VAERS, including 10 deaths.
Sept. 2, 2021: 654 reports of myocarditis or pericarditis in Pfizer's safety database, company says. Seventy-seven reports of myocarditis or myocarditis and pericarditis in Moderna's safety database, company says. Some patients, including a young male, died or had not recovered.
Sept. 2, 2021: Based on the reports and other data, a causal association between myocarditis/pericarditis and the mRNA vaccines is "considered of at least a reasonable possibility," the European Medicines Agency says.
Sept. 3, 2021: 67 myocarditis/pericarditis cases recorded in VSD among people aged 12 to 39, CDC says.
Sept. 8, 2021: Healthy, young boys face a higher risk of cardiac events from vaccines than from COVID-19, U.S. researchers find.
Sept. 9, 2021: U.S. President Joe Biden and his administration announce COVID-19 vaccine mandates for tens of millions of Americans, including many young, healthy people.
Sept. 15, 2021: Hong Kong changes recommendation from two doses of Pfizer to one dose after spike in myocarditis cases.
Sept. 17, 2021: Excess myocarditis risk for vaccinated males aged 16 or 17 "approaching 200 cases per million," FDA discloses, based on data from Optum health care claims database.
Sept. 21, 2021: "A clear signal for vaccine associated myocarditis has emerged," U.S. doctors say.
Sept. 22, 2021: One case of myocarditis reported after Pfizer booster, CDC official says. Not possible to determine the risk of rare side effects like myocarditis after boosting, CDC safety committee says.
Sept. 22, 2021: V-safe does not collect information on myocarditis, CDC official admits.
Sept. 22, 2021: FDA authorizes a Pfizer booster for millions of Americans.
Sept. 23, 2021: CDC officials say they don't know how many cases of myocarditis booster shots will cause. They project up to 26 cases per million boosters in 18- to 29-year-olds, but say more COVID-19 cases and hospitalizations would be prevented.
Sept. 24, 2021: CDC recommends Pfizer's booster for certain populations.
Sept. 27, 2021: New Zealand expert tells CDC it "seems to make huge sense to me" to delay second doses for young people.
Sept. 28, 2021: Pfizer vaccine may have "played a role" in death of 15-year-old California boy who died after receiving shot, medical examiner tells CDC.
Sept. 29, 2021: CDC meets on non-COVID vaccines.
Sept. 30, 2021: CDC falsely tells California officials that reports of postvaccination myocarditis did not come until June 2021.
October 2021: 321 cases of myocarditis, pericarditis, or myopericarditis reported to VAERS, including five deaths.
Oct. 4, 2021: Identification of postvaccination myocarditis cases "does not change clinical decision-making," JAMA Internal Medicine editors say.
Oct. 7, 2021: Norway, Finland, and Sweden suspend use of Moderna's vaccine for younger people due to myocarditis risks.
Oct. 8, 2021: Iceland suspends use of Moderna's vaccine due to the heart inflammation.
Oct. 11, 2021: CDC director briefed on how Nordic countries are limiting vaccination due to myocarditis.
Oct. 12, 2021: Secret U.S. government meeting considering counting post-infection immunity as one or more vaccine doses ends with no update.
Oct. 14, 2021: FDA says more myocarditis/pericarditis cases have been reported to its Biologics Effectiveness and Safety Initiative system.Oct. 21, 2021: The U.S. Department of Veterans Affairs has identified 7 post-vaccination myocarditis cases, CDC publicly discloses, but no signal was established and all cases resolved.
Oct. 21, 2021: Highest rate of myocarditis following vaccination, based on VAERS data, is 69.1 cases per million second shots among males aged 16 or 17, Dr. Su says (pdf).
Oct. 21, 2021: About a quarter of cases in people 29 or younger reviewed in VAERS were not known to have recovered, according to available data, with 19 still hospitalized.
Oct. 21, 2021: Analyses of VSD data "indicate that both Pfizer and Moderna are associated with increased risk of myocarditis/pericarditis in 12–39-year-olds," Kaiser Permanente doctor says (pdf). Myocarditis/pericarditis cases in VSD rise to 138, with rates higher after Moderna vaccination compared to Pfizer vaccination.
Oct. 21, 2021: CDC says tens of millions of Americans should get a Pfizer or Moderna booster.
Oct. 22, 2021: Rates of reported cases among males aged 18 to 24 approximately 139 per million after Moderna second dose and 43 per million after Pfizer second dose, French researchers say.
Oct. 22, 2021: Norway says adolescents can't get second doses of Pfizer's vaccine.
Oct. 26, 2021: FDA says benefits of vaccination outweigh the risks. Projection of harm is highest for 16- and 17-year-old males, at 196 postvaccination myocarditis/pericarditis cases, and 171 myocarditis/pericarditis hospitalizations per million doses.
Oct. 26, 2021: CDC cardiologist Dr. Matthew Oster says "we don't know a whole lot" about myocarditis after COVID-19 vaccination. "We really need to see what the long-term outcomes for these kids will be," he added later.
Oct. 26, 2021: Dr. Michael Nelson, an FDA vaccine adviser, says he's concerned that some cases of postvaccination myocarditis aren't being reported.
Oct. 26, 2021: Dr. Eric Rubin, another FDA adviser, says "we’re never going to learn about how safe this vaccine is unless we start giving it."
Oct. 27, 2021: World Health Organization says the mRNA vaccines likely cause myocarditis.
Oct. 27, 2021: Prevalence of postvaccination myocarditis may be underestimated due to its reliance on symptoms for diagnosis, researchers say.
Oct. 28, 2021: Dr. Su says myocarditis reported after COVID-19 vaccination "has been neither severe nor persistent ... however, we'll need to wait to see if longer-term complications arise." He also says CDC does not know what percent of cases became chronic.
Oct. 29, 2021: FDA authorizes Pfizer's vaccine for children aged 5 to 11 despite dearth of efficacy data.
Oct. 30, 2021: Seventeen myocarditis patients seen at a single facility in Switzerland between March and July 2021, researchers report (pdf).
Oct. 31, 2021: FDA delays decision on approving Moderna's vaccine for children.
November 2021: 267 cases of myocarditis, pericarditis, or myopericarditis reported to VAERS, including six deaths.
Nov. 1, 2021: Dr. Su says in email that "we're all doing our part to build confidence" in COVID-19 vaccines.
Nov. 1, 2021: English researchers estimate vaccinating children aged 12 to 17 will prevent 230 to 4,590 COVID-19 hospitalizations while causing 160 cases of myocarditis requiring hospitalization.
Nov. 1, 2021: Seven patients still had symptoms about a month after being diagnosed with myocarditis, U.S. researchers report.
Nov. 2, 2021: Nine deaths with myocarditis reported among people 29 and younger in VAERS. Most deaths had at least one potential non-vaccination cause while evaluation of two was still ongoing, CDC official says (pdf).
Nov. 2, 2021: Of postvaccination myocarditis patients who received cardiac MRIs, 72 percent had abnormal results, official says. Of patients who responded to CDC survey, 48 percent reported symptoms persisting after three months.
Nov. 2, 2021: Rates of myocarditis after Pfizer vaccination in children aged 5 to 11, if cleared, is unknown, CDC says (pdf). Rates in those 12 to 15 are as high as 108.5 per million second doses among males.
Nov. 2, 2021: CDC recommends virtually all children aged 5 to 11 receive a Pfizer series.
Nov. 3, 2021: 18-year-old previously healthy woman dies after COVID-19 vaccination with fulminate myocarditis, Washington state officials tell CDC. They attribute the myocarditis to asymptomatic COVID-19. Family declined an autopsy at one facility and King County Medical Examiner's Office says it did not perform an autopsy due to "social and complex" reasons.
Nov. 8, 2021: France halts Moderna's vaccine for people under 30.
Nov. 16, 2021: UK recommends second dose for 16- and 17-year-olds.
Nov. 18, 2021: Reported cases of myocarditis as high as 117 per million after doses of Moderna, and 47 per million doses of Pfizer, among males aged 18 to 29, German researchers say.
Nov. 19, 2021: Lower rates of myocarditis reported to VAERS after booster doses, CDC official says (pdf). Fifty-four reports were lodged, with some people not known to have recovered.
Nov. 19, 2021: CDC enables all adults to receive a booster of the Pfizer or Moderna vaccines.
Nov. 23, 2021: Between Dec. 27, 2020 and Sept. 3, 2021, 113 patients with suspected myocarditis after COVID-19 vaccination presented to a single practice, German researchers report.
Nov. 26, 2021: Six people were reported to have died with myocarditis or pericarditis after Pfizer vaccination, the Netherlands Pharmacovigilance Centre Lareb reports. Other causes were found for two of the deaths.
Nov. 28, 2021: Hong Kong researchers find rate of 212 myocarditis cases among 12- to 17-year-old males per million Pfizer second doses.
Nov. 28, 2021: Three verified cases of post-booster myocarditis, CDC discloses in private emails.
Nov. 29, 2021: UK recommends second dose for 12 to 15-year-olds.
December 2021: 304 cases of myocarditis, pericarditis, or myopericarditis reported to VAERS, including five deaths.
Dec. 1, 2021: 22-year-old woman spent 74 days in hospital with myocarditis after AstraZeneca vaccination, South Korean researchers report.
Dec. 2, 2021: European Medicines Agency says safety signal confirmed for postvaccination myocarditis.
Dec. 2, 2021: Israeli researchers estimate 106.9 myocarditis cases per million vaccinated males aged 16 to 29.
Dec. 9, 2021: FDA authorizes booster doses for 16- and 17-year-olds.
Dec. 9, 2021: CDC says 16- and 17-year-olds should get a booster shot. It does not mention myocarditis.
Dec. 14, 2021: 114 vaccinated people had myocarditis listed as a cause of death on their death certificate, English researchers report.
Dec. 16, 2021: Eight cases of myocarditis among children aged 5 to 11 confirmed, including one in a 6-year-old male, CDC official says.
Dec. 18, 2021: 26-year-old New Zealand man's death ruled as being caused by vaccine-induced myocarditis following an autopsy.
Dec. 25, 2021: Males under 40 face an excess risk of 101 cases per million second doses, UK researchers find.
Dec. 27, 2021: Incidence of myocarditis among males 12 to 39 is 195.4 per million second doses among males aged 12 to 39, U.S. researchers estimate. They identify cases missed by the CDC.Dec. 28, 2021: Risk of myocarditis much higher from Moderna's vaccine compared with Pfizer's shot, Canadian officials report.
Dec. 30, 2021: 21 patients with myocarditis after COVID-19 vaccination admitted to a single center between Dec. 15, 2020, and June 15, 2021, U.S. researchers report.
Dec. 30, 2021: 14-year-old hospitalized with shock after Pfizer vaccination, Moroccan doctors say.
2022
Jan. 3, 2022: Non-vaccine causes of death ruled out in death of 57-year-old New Zealand woman who experienced myocarditis after COVID-19 vaccination, researchers report.
Jan. 5, 2022: 327 myocarditis cases among children aged 12 to 15 reported to VAERS, with some cases not known to have recovered, CDC official says (pdf). Rates among males in the age group estimated at 45.7 per million second doses and 4.3 per million second doses among males aged 5 to 11, both of which were higher than expected.
Jan. 5, 2022: Most confirmed myocarditis patients aged 12 to 17 in VSD hospitalized, with more than a quarter being hospitalized for at least four days, VSD researcher says (pdf). Excess cases as high as 70.8 within 21 days per million second doses in the age group. Nearly half of the confirmed cases had new or worsening symptoms during follow-up visits at least three months later, and half had abnormal electrocardiogram results.
Jan. 5, 2022: Rate of myocarditis in Israel after Pfizer vaccination reported as high as 153 per million second doses among males aged 16 to 19.
Jan. 11, 2022: 26-year-old American man confirmed to have died from vaccine-induced myocarditis.
Jan. 19, 2022: Rates of reported cases in the United Kingdom as high as 71 per million second Moderna doses among people aged 18 to 29.
Jan. 24, 2022: CDC alerted to death of male with autopsy-confirmed, vaccine-induced myocarditis.
Jan. 25, 2022: Rates of reported myocarditis cases 70.7 per million second doses for males 12 to 15 and 105.8 per million second doses for males aged 16 and 17, CDC reports. Agency has verified 1,626 reports and says underreporting likely.
Jan. 31, 2022: FDA approves (pdf) Moderna's vaccine for people 18 and older. FDA says data on reported cases is "not sufficient to assess known serious risks of myocarditis and pericarditis and identify an unexpected serious risk of subclinical myocarditis."
Feb. 3, 2022: Death of 24-year-old American man confirmed from vaccine-induced myocarditis. Man's family later sues U.S. government.
Feb. 4, 2022: Three cases of pericarditis among vaccinated trial participants, Moderna reports (pdf). Two cases, including one in a 23-year-old male, were classified as vaccine-related.
Feb. 4, 2022: Rates of reported VAERS myocarditis cases as high as 40 per million second doses among 18- to 24-year-olds within seven days of vaccination, CDC official says. Most patients were hospitalized and discharged but 30 percent were not known to have recovered.
Feb. 4, 2022: Excess cases of myocarditis up to 61.8 per million within seven days of second Moderna doses in males, according to data from VSD.
Feb. 4, 2022: Excess risk of myocarditis 9.8 times higher after a Moderna second dose among males aged 12 to 39 when compared to the unvaccinated, Danish officials report to the CDC.
Feb. 4, 2022: Among 360 people who suffered myocarditis and responded to a CDC survey an average of 143 days afterwards, about half reported at least one symptom, such as chest pain, in the prior two weeks, CDC says (pdf). According to patient providers, about four in 10 had restrictions on physical activity months after the diagnosis.
Feb. 4, 2022: Data now show an "association of myocarditis" with Moderna's vaccine and young people, CDC safety committee says. Group says it will continue to review data on myocarditis after vaccination.
Feb. 4, 2022: Lower rates of myocarditis recorded when the space between the two primary series doses is extended, Canadian researchers tell CDC.
Feb. 4, 2022: 68 cases of myocarditis expected after each million Moderna second doses, and 47 after each million Pfizer second doses, among males aged 18 to 39, CDC says (pdf). More hospitalizations are expected to be prevented, according to the agency, keeping the benefits higher than the risks. They use outdated effectiveness assumptions and myocarditis rates from VSD.
Feb. 4, 2022: Definition of myocarditis cases expanded after feedback, VSD researcher reveals (pdf).
Feb. 9, 2022: FDA claims "the societal benefit of mass vaccination is overwhelming."
Feb. 11, 2022: FDA postpones planned meeting on whether to authorize Pfizer's vaccine for babies and toddlers.
Feb. 11, 2022: Internal Pfizer document says "there is evidence that suggests patients who receive a COVID-19 vaccine are at an increased risk of myocarditis."
Feb. 14, 2022: U.S. medical examiners report two deaths among minors from myocarditis following Pfizer vaccination.
Feb. 15, 2022: 21 patients with myocarditis after COVID-19 vaccination at a facility in Canada through November 2021, researchers report.
Feb. 23, 2022: CDC updates guidance to say people, including young males, might want to wait eight weeks between primary series doses.
Feb. 25, 2022: Hong Kong researchers report one in 2,563 males aged 12 to 17 suffered myocarditis after Pfizer vaccination.
March 10, 2022: Myocarditis rates in children aged 12 to 15 higher among vaccinated versus unvaccinated, Israeli researchers report.
March 10, 2022: "Vaccine-induced myocarditis should be considered in patients with acute symptom onset after mRNA vaccination," German researchers say.
March 15, 2022: Persistent heart injury found in followup 106 to 134 days after myocarditis diagnosis in five teenagers.
March 18, 2022: 17-year-old boy spent 23 days in hospital with myocarditis after second Pfizer dose, Japanese researchers report.
March 21, 2022: U.S. researchers estimate in preprint paper 450 myocarditis cases per million doses among males aged 12 to 17.
March 23, 2022: Heart scarring detected in seven of nine patients seven to eight months after myocarditis diagnosis, Israeli doctors report. Scarring has been associated with sudden death and patients are recommended to receive annual checkups.
March 25, 2022: CDC runs Proportional Reporting Ratio on VAERS data for the first time and detects safety signals for both the Moderna and Pfizer vaccines. The CDC falsely claimed it started the mining in 2021, but later retracted the claim.
March 25, 2022: Many patients with followup at three to eight months had persistent abnormalities on cardiac MRIs, U.S. researchers report.
March 25, 2022: Ethics experts warn against vaccinating healthy children.
April 6, 2022: Israel has recorded 213 cases of postvaccination myocarditis, official says.
April 8, 2022: Non-peer-reviewed CDC study concludes COVID-19 presents a higher risk of myocarditis than vaccination.
April 20, 2022: Myocarditis meets safety signal in VSD, CDC panel told (pdf). About half of the cases were verified through review of medical charts. Forty cases occurred after a booster in people aged 12 to 39 and another 34 occurred after a booster in those aged 40 and up.
April 20, 2022: Number of reported cases in VAERS rises to 148 within seven days of a booster, CDC official reports. The highest rate is 23.2 cases per million Pfizer booster doses among people aged 16 or 17. Most patients were hospitalized but all were discharged.
April 20, 2022: New data "do not suggest safety concerns beyond those previously identified," CDC's vaccine safety committee says (pdf).
April 20, 2022: Reported cases in U.S. Department of Veteran Affairs system up to 40; chart review confirmed 15.
April 20, 2022: Up to 379 excess cases per one million vaccinated males aged 16 to 24, Nordic researchers estimate.
April 26, 2022: FDA projects per one million Pfizer primary series, up to 196 excess myocarditis cases requiring hospitalizations will be caused among males aged 16 or 17. That number exceeds the number of prevented COVID-19 hospitalizations in two of three scenarios.
April 28, 2022: Late gadolinium enhancement (LGE), identified in 80 percent of myocarditis patients who had followup cardiac MRIs a median of 92 days after hospital discharge, U.S. researchers report. LGE is associated with sudden death.
May 2022: Proportional Reporting Ratio analyses comparing the mRNA vaccines to other vaccines is conducted by the CDC on VAERS data for the first time, showing a safety signal for myocarditis for both shots.
May 3, 2022: Subclinical pericarditis detected in one of 41 people in prospective booster study.
May 4, 2022: Signs of scarring in followup cardiac MRIs, Canadian researchers report.
May 5, 2022: FDA limits use of Johnson & Johnson's vaccine over blood clotting risks.
May 6, 2022: "There is no test or investigation that can establish causality for vaccine-associated myocarditis or pericarditis," Canadian doctors say.
May 14, 2022: Study finds Pfizer vaccine effectiveness turns negative over time in some children.
May 17, 2022: Hong Kong authorities opt to compensate family of 66-year-old woman who died from myocarditis after Pfizer vaccination.
May 19, 2022: CDC reports postvaccination myocarditis death in young boy. CDC acknowledges "rare reports" of postvaccination myocarditis deaths in other countries, but claims "it is unclear to what extent such cases were investigated."
May 19, 2022: 64 cases of myocarditis after Pfizer vaccination reported among children aged 5 to 11 in VAERS; 20 were confirmed, CDC says (pdf). Another 10 cases reported in the age group in VSD, 6 were confirmed.
May 19, 2022: Reported rates of myocarditis within seven days of vaccination as high as 74.2 per million after a second dose of Pfizer's vaccine among males 16 or 17. Rates for males are 48.1 for 12 to 15 and 35.3 for 18 to 24.
May 19, 2022: As CDC panel of advisers votes in support of Pfizer booster for ages 5 to 11, adviser Veronica McNally says parents should know rates of myocarditis are lower after a booster and that the booster will increase protection.
June 7, 2022: CDC official says (pdf) evidence supports a causal association between the mRNA vaccines, the first time the CDC has made such a statement.
June 7, 2022: Reported rates of myocarditis higher than expected in males as young as 5 and as old as 49, CDC says. The number of verified cases ending in deaths is up to 21, but CDC says none of the deaths have been confirmed as having been caused by the myocarditis.
June 7, 2022: Survey of health care providers who cared for patients estimated 18.3 percent had not fully recovered months later. Available information indicates most people "recover from myocarditis by 3-8 months after diagnosis," Dr. Shimabukuro says, but "there can be long-term effects."
June 7, 2022: Novavax says five cases of myocarditis happened in vaccinated volunteers in its COVID-19 vaccine trials.
June 11, 2022: 411 myocarditis cases detected within seven days of COVID-19 vaccination in health claims database that includes CVS Health data.
June 14, 2022: Rates of reported myocarditis following a booster higher than expected in males aged 12 to 29, CDC official says (pdf).
June 14, 2022: Myocarditis meets safety signal for children aged 12 to 17 after first and second doses in FDA's BEST system, FDA official says (pdf).
June 17, 2022: FDA authorizes Pfizer and Moderna vaccines for children as young as 6 months of age.
June 17, 2022: Hong Kong's single-dose recommendation led to a decline in myocarditis cases, researchers find.
June 21, 2022: Heart failure and sudden death recorded after COVID-19 vaccination, Hong Kong researchers report.
June 21, 2022: It is "difficult to know ... what the myocarditis rates would be" among adolescents after boosting, CDC official says.
June 23, 2022: Nearly 1,000 cases of myocarditis among children 5 to 17 after Pfizer vaccination reported to VAERS, CDC official says (pdf). Verified reports show rates as high as 75.9 per million among males 16 or 17 after a second Pfizer dose.
June 23, 2022: 37 percent of patients whose health care provider responded to a CDC survey were not confirmed to be fully recovered at least 90 days after diagnosis.
June 23, 2022: Myocarditis meets safety signal criteria in VSD for boosters from Pfizer and Moderna, CDC panel told. Excess cases as high as 200.3 per million boosters among males 16 or 17, which is higher than the second dose rate. Many patients were hospitalized but all were discharged.
June 23, 2022: Dr. Sarah Long, a CDC adviser, expresses surprise with the myocarditis rate following a booster.
June 23, 2022: More than 1,000 myocarditis reports in Moderna's database, Moderna official says (pdf).
June 23, 2022: Likely patients who suffered myocarditis after a first dose, got vaccinated again, and suffered myocarditis again, CDC official says.
June 23, 2022: Vaccinated at higher risk of serious adverse events of special interest, researchers find in reanalysis of clinical trial data.
June 25, 2022: French researchers find one myocarditis case per 5,900 males aged 18 to 24 who receive a second Moderna dose.
June 28, 2022: 75 reports of confirmed myocarditis following vaccination in adolescents aged 12 to 17 as of Feb. 22, 2022, Australian doctors report.
July 3, 2022: 27-year-old man's death 28 days after losing consciousness in sports practice and 36 days after Moderna vaccination ruled due to myocarditis after autopsy.
July 4, 2022: Prospective study of health care workers receiving a booster identifies two cases of myocarditis among about 3,800 subjects.
July 13, 2022: FDA authorizes Novavax's COVID-19 vaccine even after finding evidence that the vaccine increased the risk of myocarditis and pericarditis.
July 14, 2022: 45 myocarditis events reported in children 5 to 11, some verified, during first four months of COVID-19 vaccine administration to the age group.
July 19, 2022: Number of confirmed VAERS myocarditis reports up to 1,321, with another 515 ruled out or still under review. Highest rate remains 75.9 per million second doses administered to males 16 or 17. Highest excess case rate in VSD is 62.8 per million Moderna second doses in 18- to 39-year-old males.
July 19, 2022: CDC recommends Novavax's vaccine for adults.
July 20, 2022: German doctors call for more autopsies of people who die with myocarditis following COVID-19 vaccination.
July 20, 2022: 41 myocarditis cases, including one death, among adults following COVID-19 vaccination in Kaiser Permanente health system through Feb. 28, 2022.
July 28, 2022: 448 myocarditis or pericarditis cases identified in Italy through Sept. 30, 2021.
July 30, 2022: Excess myocarditis cases per million second doses among males aged 18 to 29 estimated at 162 and among males aged 39 to 39 estimated at 244, Canadian researchers say.
Aug. 5, 2022: CDC official admits giving false information on COVID-19 vaccine safety monitoring.
Aug. 8, 2022: Prospective study of 301 school students finds 29 percent experienced cardiovascular effects after Pfizer vaccination, Thai researchers report. "Adolescents receiving mRNA vaccines should be monitored for cardiovascular side effects," they say.
Aug. 8, 2022: 3,107 reports of myocarditis after vaccination received through Feb. 15, 2022, in Moderna's global safety database.
Aug. 16, 2022: Data mining for the Moderna and Pfizer vaccines "didn't really contribute to much of anything," CDC official says privately.
Aug. 22, 2022: More than 2,800 hospital admissions with myocarditis identified after COVID-19 vaccination, UK researchers report.
Aug. 31, 2022: FDA authorizes updated, bivalent Pfizer and Moderna vaccines as boosters for people 12 and older. The boosters had only been tested in animals.
Sept. 1, 2022: Risk of myocarditis from bivalent COVID-19 boosters is "unknown," CDC official says (pdf).
Sept. 1, 2022: CDC recommends people aged 12 and up receive one of the new boosters.
Sept. 1, 2022: No reports of myocarditis to VAERS among young children, CDC official says. Higher rates than expected, as high as 21.6 per million among males 16 or 17, within seven days of a booster for males aged 12 to 29.
Sept. 1, 2022: Myocarditis rates in VSD as high as 189 per million for males aged 16 or 17 after a booster, which is higher than the 137 per million after a second dose.
Sept. 6, 2022: Israeli officials say the postvaccination myocarditis cases "suggest a causal relationship" between the mRNA vaccines and the heart inflammation.
Sept. 6, 2022: U.S. Defense Secretary Lloyd Austin not alerted to myocarditis after COVID-19 vaccination through May 25, 2021, military says.
Sept. 23, 2022: Most myocarditis patients had recovered at followup 60 to 180 days after diagnosis, though some had persistent symptoms, Hong Kong researchers report.
Sept. 28, 2022: Blood samples taken during and two to four days after fourth vaccine dose showed myocardial injury in two participants, prospective study in Israel finds.
Oct. 4, 2022: Rate of reported myocarditis cases in VAERS higher after booster for young males than after second dose, CDC officials disclose.
Oct. 7, 2022: Florida's surgeon general recommends against mRNA COVID-19 vaccines for males aged 18 to 39 due to analysis of death certificates.
Oct. 15, 2022: Two of five adult myocarditis patients had persistent symptoms at followup at least 57 days after initial cardiac MRI, Minnesota researchers report.
Oct. 26, 2022: Myocarditis safety signal after Pfizer vaccination identified in people aged 12 to 64 in health claims surveillance system, FDA reports.
Oct. 31, 2022: Six of nine myocarditis patients showed persistent heart injury, with one experiencing persistent symptoms, at 12-week followup, Italian researchers report.
Nov. 4, 2022: Nine cases of myocarditis or pericarditis reported after bivalent vaccination, CDC says.
Nov. 7, 2022: Expected rate of myocarditis 269.5 cases per million Moderna second doses and 58 cases per million Pfizer second doses in males 18 to 29, Canadian researchers report.
Nov. 9, 2022: LGE still observed at followup in myocarditis patients, with two reporting persistent symptoms, German researchers report.
Nov. 12, 2022: Number of Moderna doses needed to harm higher than number of doses needed to treat for 16- to 19-year-old men, Italian researchers estimate.
Nov. 21, 2022: 141 myocarditis cases within 21 days of COVID-19 vaccination through March 10, 2022 in British Columbia, Canadian researchers report.
Nov. 27, 2022: Autopsies of German people who died unexpectedly within 20 days of a COVID-19 vaccination identified myocarditis in five people, with other possible causes ruled out.
Dec. 5, 2022: U.S. researchers estimate that for every COVID-19 hospitalization prevented by vaccine boosters, 1.5 to 4.6 myocarditis cases would be caused in young males.
Dec. 16, 2022: Clusters of myocarditis cases after second Pfizer dose in 12 to 39 age group in VSD, researchers with the CDC and other institutions report.
Dec. 28, 2022: Most studies on postvaccination myocarditis lacked proper stratification, review finds.
Dec. 28, 2022: LGE present in all seven myocarditis patients with followup cardiac MRIs three to six months after diagnosis, Turkish researchers report. Two patients had heart swelling.
Dec. 31, 2022: Pfizer fails to meet deadline for post-approval myocarditis study. FDA later reveals it allowed Pfizer to delay the deadline.
2023
Jan. 3, 2023: Eight sudden cardiac deaths in Qatar after COVID-19 vaccination determined to likely be from the vaccination.
Jan. 4, 2023: Analysis of blood finds "unbound" spike protein in plasma of people with myocarditis after vaccination.
Jan. 5, 2023: One percent of electrocardiograms return abnormal in prospective study of second Pfizer dose in high school students in Taiwan.
Jan. 5, 2023: "Unequivocal" evidence supporting a link between the COVID-19 vaccines and myocarditis, Portuguese Society of Cardiology says.
Jan. 9, 2023: Imaging study of five children three to 109 days after onset of myocarditis symptoms following Pfizer vaccination finds signs of heart scarring.
Jan. 26, 2023: Safety signal for myocarditis and pericarditis for adults aged 18 to 35 for Pfizer's updated vaccine, FDA official says (pdf).
Feb. 1, 2023: 530 people across Nordic countries diagnosed with myocarditis related to mRNA vaccination, researchers report. Six died.
Feb. 8, 2023: Taiwanese researchers estimate 126 myocarditis cases per million second doses among males aged 12 to 17.
Feb. 13, 2023: England stops offering younger, healthy people booster shots.
Feb. 14, 2023: 134 cases of heart failure, six cases of myocarditis in 2021 among vaccinated in 99 counties, U.S. researchers report in medical chart review.
Feb. 15, 2023: Australia found that myocarditis contributed to the death of a 21-year-old woman, newly disclosed documents show.
Feb. 22, 2023: Singapore says it will pay family of man who died with myocarditis after COVID-19 vaccination.
Feb. 24, 2023: CDC estimates a small number of myocarditis cases for every million boosters in its latest risk-benefit analysis (pdf). Experts criticize calculus as flawed.
Feb. 24, 2023: One case of myocarditis after bivalent vaccination detected in VSD, CDC official says.
Feb. 24, 2023: Risk of myocarditis after a bivalent shot is unknown, CDC official says.
Feb. 24, 2023: CDC officials give false information on vaccine safety monitoring and refuse to correct the record.
Feb. 27, 2023: Family of young girl who died from myocarditis after Pfizer vaccination to be compensated, Taiwanese authorities say.
March 3, 2023: CDC discloses it never acted on plans for a collaboration to "stimulate VAERS reporting" from health care workers and long-term care facilities."
March 9, 2023: Persistent heart inflammation identified in three of 17 people at two month followup, Canadian researchers report.
March 14, 2023: FDA authorizes Pfizer's bivalent as a booster for children under 5.
March 15, 2023: FDA adds warning about myocarditis to fact sheets for Johnson & Johnson's vaccine.
March 17, 2023: CDC adds language to its website saying the mRNA vaccines cause myocarditis.
March 20, 2023: 14-year-old girl's death was diagnosed as vaccine-related myocarditis, Japanese researchers report.
March 22, 2023: Moderna CEO Stephane Bancel claims all age groups face a higher risk of myocarditis after COVID-19 infection versus vaccination.
March 30, 2023: World Health Organization says additional boosters would provide "marginal" benefit to healthy children and younger adults.
April 3, 2023: Extending interval between primary series doses reduced myocarditis incidence, Hong Kong researchers report.
April 5, 2023: 173 postvaccination myocarditis cases among children between July 19, 2021, and Sept. 30, 2022, South Korean researchers report.
April 10, 2023: Switzerland opts against recommending COVID-19 vaccines for citizens due to high levels of previous infection and/or vaccination.
April 14, 2023: Woman died from myocarditis after COVID-19 vaccination, coroner in Singapore concludes.
April 15, 2023: Malaysian researchers identify 87 myocarditis cases within 21 days of COVID-19 vaccination among those vaccinated between Feb. 1, 2021, and Feb. 28, 2022.
April 18, 2023: FDA replaces old Moderna and Pfizer vaccines with the updated bivalent shots, despite no clinical trial efficacy estimates being available.
April 18, 2023: U.S. government compensates two people for myocarditis injuries caused by COVID-19 vaccines.
April 18, 2023: FDA rejects request to add risk of sudden death to labels for mRNA vaccines.
April 19, 2023: CDC officials, including Dr. Shimabukuro, repeat lies about COVID-19 vaccine safety monitoring.
April 20, 2023: CDC says unvaccinated people should get a bivalent, and some vaccinated people should get an additional shot.
April 24, 2023: mRNA COVID-19 vaccines did not have an effect on overall mortality, researchers say after crunching trial data.
May 10, 2023: Some myocarditis patients had LGE on cardiac MRIs performed around 107 days after initial MRIs, U.S. researchers report.
May 11, 2023: COVID-19 public health emergency ends, but vaccines are still available under emergency authorization.
May 11, 2023: 96 percent of cases registered as postvaccination myocarditis verified as correct registration, Swedish researchers report.
May 12, 2023: Most remaining federal COVID-19 vaccine mandates end.
May 22, 2023: FDA says myocarditis hits safety signal criteria in children aged 12 to 17.
May 22, 2023: Vaccine-injured sue federal government over Big Tech censorship.
June 1, 2023: U.S. government compensates third person for myocarditis caused by COVID-19 vaccination.
June 1, 2023: FDA revokes authorization of Johnson & Johnson's COVID-19 vaccine.
June 2, 2023: Sudden deaths caused by COVID-19 vaccination, South Korean researchers determine.
June 13, 2023: CDC claims no postvaccination deaths have been caused by myocarditis.
June 13, 2023: South Korean researchers estimate 82 myocarditis per million third doses in those under 19.
June 14, 2023: Seniors not at higher risk of myocarditis after COVID-19 vaccination, FDA reports in review of surveillance data.
June 15, 2023: FDA estimates that each one million Moderna primary series among people aged 18 to 25 will prevent 635 to 5,957 COVID-19 hospitalizations, and cause an excess of 68 to 241 myocarditis cases.
June 27: 139 postvaccination myocarditis cases across 29 centers in Spain from Aug. 1, 2021, to March 10, 2022, researchers report. After a 3-month follow-up, the incidence of adverse events was 12.7 percent, with 1.4 percent mortality.
June 30, 2023: Deadline for Moderna's subclinical myocarditis study passes without publication.
July 17, 2023: Australia stops reporting postvaccination myocarditis cases, saying rates have stabilized.
July 20, 2023: One in 35 health care workers had signs of heart injury associated with vaccination, prospective study finds.
July 31, 2023: Long-term issues detected in followup of up to one year, Hong Kong researchers report.
Aug. 3, 2023: Pfizer officials tell Australian Senate it doesn't know why company's vaccine inflames the heart.
Sept. 5, 2023: CDC repeatedly told people who suffered adverse events after vaccination to get additional shots, documents show.
Sept. 12, 2023: Two cases of myocarditis or pericarditis after bivalent vaccination in VSD, CDC officials report.
Sept. 12, 2023: Bivalent protection against hospitalization sharply drops within months, CDC data show.
Sept. 12, 2023: CDC recommends new vaccines with trial data from just 50 people.


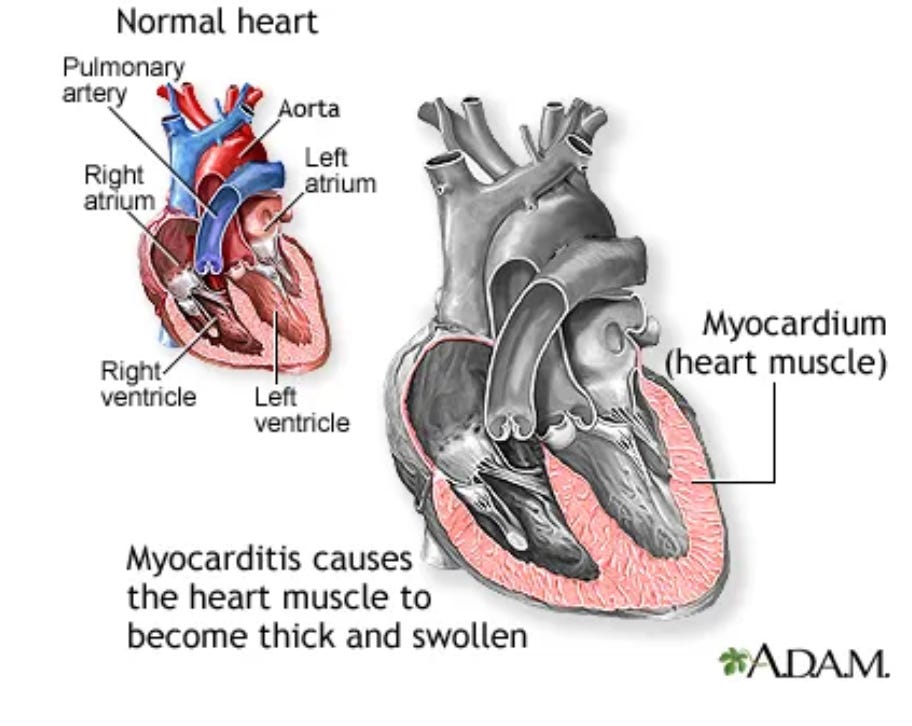








A fallacious argument was presented and probably believed by officials that if a person took the vaccine they might run the risk of heart effects but would be saved from possible death from Covid. This is not informed consent. The vast majority of potential vaccinees were healthy people under the age of 65 who probably had no risk of death or serious illness from Covid, particularly when good treatments are available. But by taking the vaccine they acquired a definite risk of heart damage. It was irrational for such people to take the vaccine.
Re advised consent.... also seems very derelict in their responsibility to patients not to warn those allergic to ethylene glycol to its presence in these jabs.