Revised HHS Childhood Vax Schedule Announced
Changes stem from reviewing practices in other nations, including consulting experts in Denmark, Germany, and Japan.
Overview
After a scientific review of the underlying science, comparing the U.S. child and adolescent immunization schedule with those of peer, developed nations, Centers for Disease Control and Prevention Acting Director Jim O’Neill has now further updated the U.S. childhood immunization schedule. The changes stem from reviewing practices in other nations, including consulting experts in Denmark, Germany, and Japan.
The CDC will, moving forward, stop broadly recommending vaccines against influenza, rotavirus, respiratory syncytial virus, hepatitis A, and meningococcal disease. The CDC in 2025 already narrowed recommendations for hepatitis B and COVID-19 vaccination, based on guidance from ACIP advisers selected by HHS Secretary Kennedy.
The CDC will continue to recommend that all children are immunized against 10 diseases for which there is international consensus, as well as varicella (chickenpox). For other diseases, the CDC will recommend immunization for high-risk groups and populations, or through shared clinical decision making when it is not possible for public health authorities to clearly define who will benefit from an immunization. The updated schedule is in contrast to the CDC child and adolescent schedule at the end of 2024, which recommended 17 immunizations for all children.
The Danish childhood vaccination schedule that has influenced this new US schedule is far simpler, slower, and gentler than the U.S. CDC schedule. Denmark begins immunization at three months of age, giving only about a dozen total injections by adolescence, focused on serious diseases such as diphtheria, tetanus, polio, Hib, measles, and meningitis.
The U.S. begins vaccination the day a child is born, in some cases with a hepatitis B shot, and continues frequently through infancy, totaling around sixty doses by the end of adolescence. The Danish program avoids vaccines for mild illnesses like chickenpox, rotavirus, and hepatitis A, and it does not recommend annual flu or early COVID inoculations for healthy children. It therefore introduces far fewer antigens, adjuvants, and chemical additives, giving the immune system more time to mature between doses.
The U.S. system, by contrast, compresses numerous injections into the first year of life, creating heavy antigen and aluminum exposure during a critical developmental window. Denmark’s approach reflects a minimalist “target the serious diseases” philosophy built on transparency and trust, while the U.S. program embodies a maximalist “vaccinate for everything” model driven by a zero‑risk culture, liability avoidance, institutional inertia, and a cult-like belief that all vaccine products are “safe and effective” and therefore above questioning. Both countries maintain high vaccine coverage, but Denmark achieves comparable disease control with a fraction of the biochemical and immunological load imposed on young children in the United States.
The updated CDC childhood immunization schedule:
1. Recommends all vaccines for which there is consensus among peer nations.
2. Allows for more flexibility and choice, with less coercion, by reassigning non-consensus vaccines to certain high-risk groups or populations and shared clinical decision-making.
3. Ensures that all the diseases covered by the previous immunization schedule will still be available to anyone who wants them through Affordable Care Act insurance plans and federal insurance programs, including Medicaid, the Children’s Health Insurance Program, and the Vaccines for Children program. Families will not have to purchase them out of pocket. Among peer nations, the U.S. will continue to offer the most childhood vaccines for free to those who wants them.
4. Is accompanied by a strengthening of vaccine research through HHS’ commitment to double-blind placebo controlled randomized trials as well as more observational studies to evaluate long-term effects of individual vaccines and the vaccine schedule.
Scientific Review
In 2024, the U.S. recommended more childhood vaccine doses than any other peer nation, and more than twice as many as some European nations. A 2024 comparison between the U.S. and peer nations, found that countries without vaccine mandates had as high immunization rates as the U.S. and other countries with vaccine mandates.
Trust in U.S. public health declined from 72% to 40% between 2020 and 2024, coinciding with public health failure during the pandemic, including COVID-19 vaccine mandates. Though the COVID-19 vaccine was recommended for all children on the CDC schedule, the uptake rate was less than 10% by 2023. The uptake rate of other childhood vaccines declined during the same time period.
Large placebo-controlled randomized trials on individual vaccines, combinations of vaccines, and vaccine schedules, as well as observational studies, are needed to better inform patients, parents, and providers and help restore trust in public health.
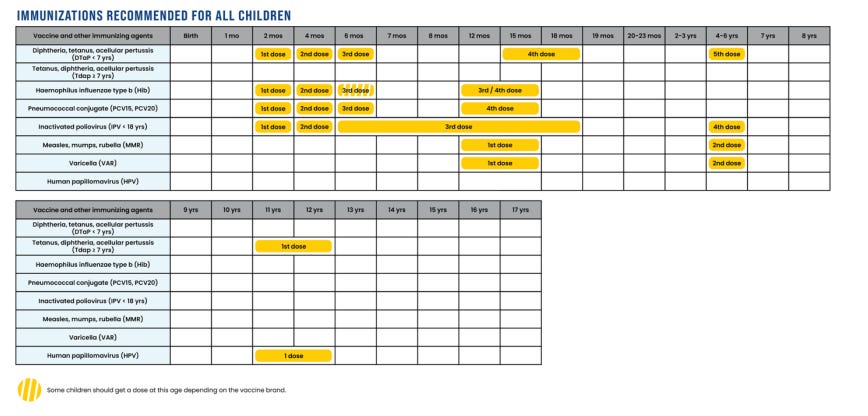
Immunizations Recommended for All Children
The CDC will continue to recommend that all children are vaccinated against diphtheria, tetanus, acellular pertussis (whooping cough), Haemophilus influenzae type b (Hib), Pneumococcal conjugate, polio, measles, mumps, rubella, and human papillomavirus (HPV), for which there is international consensus, as well as varicella (chickenpox).
Recent scientific studies have shown that one dose of the HPV vaccine is as effective as two doses. The CDC is following the lead of several peer nation by recommending one instead of two doses of this vaccine.
The updated CDC recommended immunizations for all children and adolescents will maintain robust protection against diseases that cause serious morbidity or mortality to children.
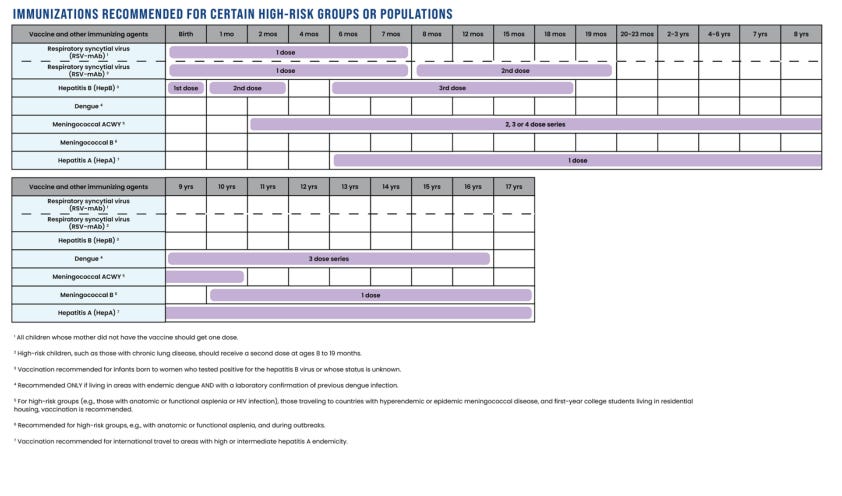
Immunizations Recommended for Certain High-Risk Groups or Populations
Like all medical products, vaccines and other immunizing agents have different risk-benefit profiles for different groups of people. Risk factors can include unusual exposure to the disease, underlying comorbidities, or the risk of disease transmission to others.
The immunizations recommended for certain high-risk groups or populations are for respiratory syncytial virus (RSV), hepatitis A, hepatitis B, dengue, meningococcal ACWY, and meningococcal B.
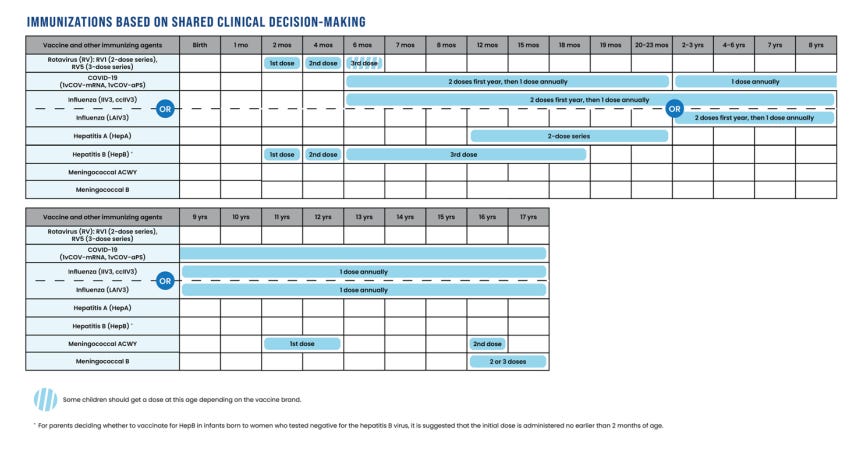
Immunizations Based on Shared Clinical Decision-Making
It is not always possible for public health authorities to clearly define who will benefit from an immunization, who has the relevant risk factors, or who is at risk for exposure. Physicians and parents, who knows the child, are then best equipped to decide based on individual characteristics.
The immunizations based on shared clinical decision-making are for rotavirus, COVID19, influenza, meningococcal disease, hepatitis A, and hepatitis B.
Insurance Coverage
All immunizations recommended by the CDC as of December 31, 2025, will continue to be fully covered by Affordable Care Act insurance plans and federal insurance programs, including Medicaid, the Children’s Health Insurance Program, and the Vaccines for Children program. Families will not have to purchase them out of pocket.
This means that insurance will continue to cover more vaccines for children in the U.S. than in peer nations, where insurance generally only pays for recommended vaccines.
Next Steps
For health care providers, the CDC will publish the updated Child and Adolescent Immunization Schedule by Age (through age 18) of immunization recommendations for all children, immunization recommendations for certain high-risk groups or populations, and immunizations based on shared clinical decision-making.
HHS will work with states and physician groups to educate parents and providers on the updated CDC childhood immunization schedule.
The CDC will continue to closely monitor vaccine uptake, infectious disease rates and vaccine safety.
In Conclusion
The updated U.S. childhood immunization schedule represents a decisive shift toward scientific restraint, transparency, and individualized care. By aligning more closely with peer nations such as Denmark, the new schedule narrows universal recommendations to vaccines with clear international consensus against the most serious childhood diseases, while reclassifying others, such as influenza, rotavirus, and hepatitis A, for high‑risk groups or shared clinical decision‑making.
This balance preserves access and affordability, ensuring that all vaccines remain fully covered by federal programs while reducing unnecessary exposure to antigens and adjuvants in early life. The reform addresses the erosion of public trust that followed overreach and coercive mandates during the pandemic years and acknowledges that vaccination, like any medical intervention, carries individualized benefit‑risk trade‑offs. Strengthening independent research through placebo‑controlled trials and long‑term safety studies will help restore scientific credibility and public confidence. Ultimately, the modernized schedule protects children from the most dangerous infectious diseases while promoting informed parental choice, evidence‑based decision‑making, and renewed trust in American public health.
Assessment of the U.S. Childhood and Adolescent Immunization Schedule Compared to Other Countries January 2, 2026
Tracy Beth Høeg, MD, Ph.D., Acting Director for the Center for Drug Evaluation and Research and FDA Ex Officio member to ACIP, and Martin Kulldorff, Ph.D., Chief Science and Data Officer for the Assistant Secretary for Planning and Evaluation, in consultation with experts at CDC, FDA, NIH, and CMS.
Executive Summary
On December 5, 2025, President Trump issued a Presidential Memorandum1 directing the Secretary of the U.S. Department of Health and Human Services (HHS) and the Acting Director of the Centers for Disease Control and Prevention (CDC) to review best practices from peer, developed nations regarding childhood vaccination recommendations and the scientific evidence underlying those practices. The President instructed them to update the U.S. core childhood vaccine schedule if they determine that superior practices exist abroad. This assessment is a scientific, evidence-based, data-driven response to the President’s directive. It argues that a change in the U.S. childhood vaccine schedule is necessary. It compares the U.S. with peer nations, examines vaccine uptake and trust, addresses clinical and epidemiological considerations and knowledge gaps, analyzes vaccine mandates, and outlines recommendations and next steps for immediate and long-term action. The U.S. is a global outlier among peer nations in the number of target diseases included in its childhood vaccination schedule and in the total number of recommended vaccine doses.
The Acting CDC Director should immediately consider updating the Childhood Immunization Schedule to keep vaccines for 10 diseases—measles, mumps, rubella, polio, pertussis, tetanus, diphtheria, Haemophilus influenzae type B (Hib), pneumococcal disease, and human papillomavirus (HPV)—for which peer, developed nations share international consensus, as well as varicella (chickenpox) (the “consensus vaccines”) in the category of vaccines recommended for all children. These consensus vaccines will represent the core childhood vaccine schedule. This report does not substantively address the consensus vaccines. All other vaccines currently on the U.S. schedule (the “non-consensus vaccines”) should be recommended for high-risk groups and populations and/or through shared clinical decision-making, by taking individual patient characteristics into account (Figure 1). No vaccine should be moved to the “not recommended” category.
Therefore, the Acting CDC Director should update the childhood immunization schedule to reflect these three distinct components:
Figure 1: Proposed immunization schedule from birth up to age 18, with recommendations for all children (yellow), recommendations for high-risk groups or populations (purple), and immunizations with shared clinical decision-making (blue), all available and covered by insurance for all children and adolescents without cost sharing.
Importantly, all immunizations recommended by the CDC at the end of 2025—and covered by insurance at that time—should remain covered by insurance without cost sharing, as they should all remain on the schedule. This would include all diseases covered by the 2025 childhood and adolescent immunization schedule. By comparison, in many European countries, vaccines not recommended for routine use are typically not covered. By distinguishing non-consensus vaccines as options based on individual risk factors, we can better define those risk factors and identify the children who are most likely to have a net-benefit from these vaccines. Between 2020 and 2024, trust in health care declined steeply from 71.5% to 40.1%, coinciding with school closures, other lockdowns, mandatory face masks, COVID-19 vaccination mandates with their de facto denial of infection acquired immunity, and other public health recommendations that lacked scientific rationale and went against basic principles of public health. The distrust of public health agencies during the pandemic has spilled over to other recommendations made by these agencies, including those with respect to vaccines. Over the same period, there was a decline in childhood vaccination rates across the country, with, for example, a reduction in measles, mumps, and rubella (MMR) vaccination from 95.2% to 92.7%. This has increased the potential risk for measles cases. Instead of implementing vaccination mandates, most peer nations maintain high childhood vaccination rates through public trust and education. This is in contrast to the U.S., where individual states can set mandatory vaccination requirements for children as a requirement to attend school. Among the fundamental principles of public health are respect for personal autonomy and self-determination, and informed consent is a cornerstone of medical care. While vaccine mandates may increase short-term vaccination rates, coercive measures can also have negative consequences on trust that may decrease long-term vaccination rates for consensus vaccines. Increased emphasis on personalized medicine would improve informed consent and the doctor patient relationship, leading to a better educated and empowered public. The increased emphasis on shared clinical decision-making would help restore trust in public health recommendations made by CDC.
Vaccines are intended to benefit children by preventing them from contracting certain infectious diseases. But, like all medicines, vaccines come with risk that must be balanced against their benefits. Before and after licensure, manufacturers have inadequate incentives to study vaccine adverse effects. Regulatory bodies, including the FDA and the CDC have sometimes been slow to identify adverse effects in post-market studies. Vaccine safety and risks are therefore often poorly characterized, quantified, or understood. Scientifically valid rates of adverse events are rarely available to determine the relationship, if any, between our country’s immunization schedule and the increasing prevalence of chronic diseases in American children. Medical interventions given to healthy children to prevent diseases, rather than treat or cure them, should have the highest standard of safety pre- and post-marketing.
To address the safety concerns regarding the child and adolescent immunization schedule, HHS should fund gold standard scientific research, including large placebo-controlled randomized trials as needed, both on individual vaccines, combinations of vaccines, and vaccination schedules. Putting aside the ethics of administering a medical product that was not properly trialed to affirm its safety, this can be ethically accomplished by, among other methods, randomizing the timing of vaccination. HHS should also fund and conduct observational studies concerning long-term chronic adverse effects of both individual vaccines and the immunization schedule. This will better inform patients/parents and physicians moving forward, increase trust in public health, and improve the state of vaccine science globally. This assessment only considers immunizations that CDC recommended for all children at the end of 2024. It does not consider the timing or order of vaccines, nor the number of doses, with the exception of the HPV vaccine. Bringing the U.S. pediatric immunization schedule in line with the consensus of peer nations while keeping non-consensus vaccines available for high-risk groups and populations and/or through shared clinical decision-making is a balanced approach to reform and restore trust in public health. Coupled with an evaluation of potential vaccine harms, this reform seeks to restore public confidence, provide much-needed clarity for parents of young children, and preserve the benefits of immunization programs.



I look forward to the day when vaccine science is done the way other science is done, by the scientific method -- and not by consensus. Consensus seems more of an excuse or a fig leaf than a rationale for policy. HHS has made some progress here, and deserves credit. Perhaps politically speaking, doing science based policy in a "Vaccines Amen!" culture is a bridge too far just yet. I hope we can one day soon cross that bridge to the land of science. I will truly say Amen to that!
A step in the right direction. This statement is accurate that has been expressed as indicated that has overbearing influences that the vaccine manufacturers have on the decision makers at the CDC and the FDA that must be corrected!
The U.S. system, by contrast, compresses numerous injections into the first year of life, creating heavy antigen and aluminum exposure during a critical developmental window.